Chapter 66 Lung Cancer
Clinical Evaluation and Staging
Symptomatic Presentation
Symptoms and Signs Related to the Primary Tumor
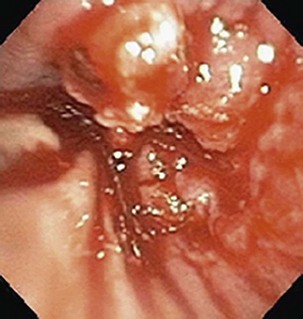
Currently, most patients with lung cancer have advanced disease at the time of presentation and are likely to present with symptoms, particularly if local extension, metastasis to distant sites, or a paraneoplastic syndrome is present. Obtaining a thorough history and a review of systems in patients suspected of having lung cancer constitute an important part of the initial evaluation, because the symptom history will influence the choice of imaging studies, the interpretation of those studies, and the institution of early palliative interventions. Symptoms and signs related to the primary tumor most commonly include cough, dyspnea, chest pain, and hemoptysis. Cough is present in up to 75% of patients with lung cancer and may be related to airway involvement, postobstructive pneumonitis, or bronchorrhea. Dyspnea may be associated with primary site–related problems, such as endobronchial or extrinsic airway obstruction, postobstructive atelectasis, infection, or increased airway secretions, or may be related to metastatic disease, with potential manifestations including pleural effusion, lymphangitic tumor spread, pericardial effusion with tamponade, and pulmonary thromboemboli in the setting of hypercoagulability. Hemoptysis with lung cancer usually manifests as intermittent or persistent bloody streaking of the sputum and rarely can be massive. In up to 9% of patients with hemoptysis and lung cancer, the chest radiograph will be normal in appearance, underscoring the need to include endobronchial tumor as a major consideration in the differential diagnosis in patients presenting with hemoptysis and lung cancer risk factors (Figure 66-1). With the exception of hemoptysis, these pulmonary symptoms may not necessarily prompt a patient with a history of cigarette use, chronic obstructive pulmonary disease, or interstitial lung disease to seek specific medical attention or, conversely, for the clinician to consider an alternative diagnosis. These may be contributing factors to the consistent observation of a delay of several months between the onset of symptoms and a definitive diagnosis of lung cancer.
Symptoms Related to Metastatic Spread
Paraneoplastic Syndromes
Paraneoplastic syndromes occur in approximately 10% of patients with lung cancer (Table 66-1). Such syndromes may be the initial presenting complaint triggering an evaluation but also can develop late in the course of disease. Paraneoplastic syndromes are unrelated to direct invasion or distant spread of tumor and in and of themselves do not preclude curative-intent therapy. Endocrine syndromes associated with lung cancers often are characterized by tumor production of biologically active hormones. Lung cancer is the most common cause of cancer-associated hypercalcemia, hyponatremia, and syndromes involving ectopic production of adrenal corticotropic hormone (ACTH).
Table 66-1 Paraneoplastic Syndromes Associated with Lung Cancer
| Endocrine | Syndrome of inappropriate secretion of antidiuretic hormone (SIADH)/hyponatremia; hypercalcemia; ectopic adrenocorticotropic hormone (ACTH) syndrome; Cushing syndrome; hyperglycemia; hypoglycemia; hyperthyroidism; carcinoid syndrome; gynecomastia; elevated growth hormone; elevated follicle-stimulating hormone (FSH); galactorrhea |
| Musculoskeletal | Hypertrophic osteoarthropathy (HOP); clubbing, myopathy; dermatomyositis; polymyositis |
| Neurologic | Lambert-Eaton myasthenic syndrome (LEMS); encephalomyelitis/subacute sensory neuropathy; cerebellar degeneration; opsoclonus-myoclonus; autonomic neuropathy; retinopathy; mononeuritis multiplex; peripheral neuropathy; myopathy |
| Skin | Acanthosis nigricans; pruritus and urticaria, erythema multiforme; erythroderma; exfoliative dermatitis; hyperpigmentation |
| Hematologic | Anemia, thrombocytosis, leukocytosis, hypercoagulable state |
Nonendocrinologic extrapulmonary syndromes include musculoskeletal abnormalities, most commonly asymptomatic digital clubbing, which can be seen in isolation or in the setting of hypertrophic pulmonary osteoarthropathy (HPO). The pathogenesis of HPO is unknown, but the disorder consists of a proliferative periostitis characterized by symmetric, painful arthropathy that typically involves the ankles, shins, knees, wrists, and elbows. The diagnosis usually is confirmed by the identification of new periosteal bone formation on plain radiographs of the long bones, or by demonstration of diffuse long bone uptake of radionuclide on bone scan or positron emission tomography (PET) imaging. HPO is more commonly seen with adenocarcinoma. Hematologic dyscrasias commonly occurring in patients with lung cancer include anemia, which may compound fatigue and dyspnea; thrombocytosis; and leukocytosis. Eosinophilia is seen only rarely. Lung cancer is the most common cause of hypercoagulability associated with malignancy, which usually declares itself as deep venous thrombosis or thromboembolism, or with classic Trousseau syndrome (migratory superficial thrombophlebitis). Lung cancer, and specifically SCLC, also is the most common cause of a clinically diverse group of paraneoplastic neurologic syndromes (see Table 66-1).
Asymptomatic Presentation
The Solitary Pulmonary Nodule
In evaluating SPNs, it is useful to distinguish small (8 mm or less in diameter) from larger nodules. Small SPNs are very common findings in patients who have participated in CT screening studies for lung cancer, as well as in those undergoing CT scanning for non-lung-related reasons, as noted earlier. In the feasibility study for the NLST performed within the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, 21% of subjects had abnormalities identified on the baseline screening CT scan, most of which were small SPNs. Similarly, in the Mayo Clinic lung cancer screening study, 51% of subjects had abnormalities found on the baseline screening study, with an increase to 69% by the second annual screen, the vast majority of which were small SPNs. The enormous number of small SPNs generated by an increased volume of CT scanning prompted a position statement from the Fleischner Society proposing guidelines for the management of small (8 mm or less) SPNs detected on CT scans. The recommendations are outlined in Table 66-2. Appropriately applied, the guidelines outline algorithms for patients based on their risk of lung cancer, providing timelines for follow-up of incidentally discovered nodules that minimize the number of CT scans necessary in the course of evaluation. These practical recommendations will become even more relevant if CT screening for lung cancer becomes the standard of practice. Of note, these recommendations apply only to patients in whom SPNs are discovered incidentally, unrelated to any known underlying disease, and only for solid SPNs 8 mm or less in diameter. Specifically, the guidelines are not intended to apply to patients known to have or suspected of having malignant disease, patients younger than 35 years of age, or patients with unexplained fever. Furthermore, the recommendations are not fully applicable to persons with nonsolid (ground glass appearance) or partially solid nodules, who may require longer follow-up to permit exclusion of biologically indolent cancers with greater confidence.
Table 66-2 Fleischner Society Guidelines for Management of Small Pulmonary Nodules Detected on Computed Tomography (CT) Scans
| Nodule Size* | Low-Risk Patient† | High-Risk Patient‡ |
|---|---|---|
| ≤4 mm | No follow-up needed§ | Follow-up CT at 12 months; if unchanged, no further follow-up¶ |
| >4-6 mm | Follow-up CT at 12 months; if unchanged, no further follow-up¶ | Initial follow-up CT at 6-12 months, then at 18-24 months if no change¶ |
| >6-8 mm | Initial follow-up CT at 6-12 months, then at 18-24 months if no change | Initial follow-up CT at 3-6 months, then at 9-12 months and 24 months if no change |
| >8 mm | Follow-up CT at around 3, 9, and 24 months, then dynamic contrast-enhanced CT, PET, and/or biopsy | Same as for low-risk patient |
NOTE: Guidelines refer to newly detected indeterminate nodules in persons 35 years of age or older.
PET, positron emission tomography.
* Average of length and width.
† Minimal or absent history of smoking and of other known risk factors.
‡ History of smoking or of other known risk factors.
§ The risk of malignancy in this category (<1%) is substantially less than that determined with a baseline CT scan in an asymptomatic smoker.
¶ Nonsolid (ground glass) or partly solid nodules may require longer follow-up period to exclude indolent adenocarcinoma.
Modified from MacMahon H, Austin JH, Gamsu G, et al: Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society, Radiology 237:395–400, 2005.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree