% presentation [6]
% late [7]
Cough
79
40
Breathlessness
5
69
Pain
55
64
Anorexia
45
70
Malaise
47
Hemoptysis
35
Hoarseness
11
Dysphagia
7
Vomiting
25
Constipation
42
Mouth problems
46
Insomnia
40
Confusion
33
Low mood
49
While most of these reflect local disease, some, such as extrathoracic pain, suggest distant metastatic spread, and malaise and anorexia are systemic manifestations of cancer and probably paraneoplastic in origin.
Another study examined the symptoms of lung cancer patients in their last year of life and compared them with those of patients with chronic obstructive pulmonary disease (COPD) [7]. Whilst some local symptoms, such as cough, improved throughout the course of the disease, others, such as pain, became more troublesome. Systemic symptoms and low mood were common in both advanced lung cancer and COPD (Table6.1).
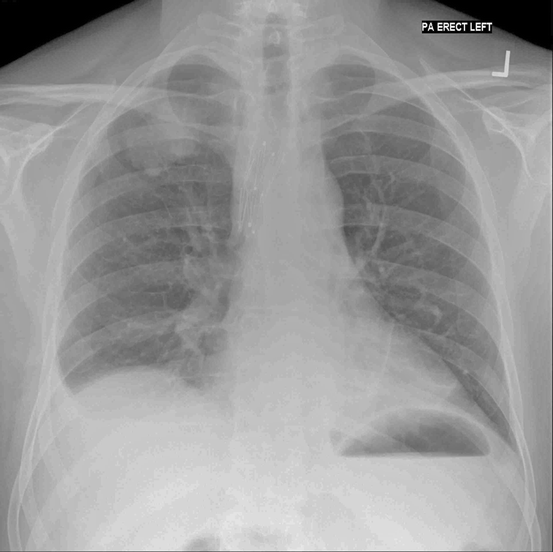
Fig. 6.1
Plain chest radiograph showing bronchial stents positioned in the right main bronchus and bronchus intermedius. The primary tumour is visible in the right upper zone and there is a small pleural effusion
Mechanism of Symptoms
Cough
Stimulation of the irritant receptors in the central airways causes a cough relatively early in patients with lung cancer. The problem is that most of these people will have been smokers and possibly have a chronic cough due to co-existent COPD. The change in the nature of the cough may be an important clue. Sputum production is not discriminating except in bronchoalveolar cell carcinoma (multifocal lepidic adenocarcinoma) which is sometimes characterized by excessive secretions (bronchorrhea).
Breathlessness
Breathlessness, like cough, is a common symptom in the patient group who develop lung cancer. The commonest underlying causes are collapse of a lobe or whole lung, and pleural effusion. Less common causes include pericardial effusion, lymphangitis carcinomatosa, superior vena cava obstruction (SVCO), tumor bulk and mediastinal involvement causing a phrenic nerve palsy with diaphragmatic paralysis.
Stridor and Hoarseness
Stridor is a predominantly inspiratory wheezing sound due either to an intra-luminal tumor narrowing a large airway or from extrinsic compression of an airway by tumor mass or lymph nodes. The wheezing sound is from turbulent airflow through the narrowing. Hoarseness is usually due to left recurrent laryngeal nerve palsy from a tumor in the left upper mediastinum. This results in paralysis of the left vocal cord. In addition to a hoarse voice, such patients also develop a “bovine” cough as they are unable to completely adduct the vocal cords to generate adequate pressure for a normal cough. Rarely a tumor at the extreme right apex causes right cord palsy.
Hemoptysis
This is an alarming symptom and therefore one likely to make the patient seek medical advice. It is usually due to mucosal disruption from a central tumor or from a peripheral tumor outgrowing its blood supply and cavitating. Major hemoptysis is uncommon at presentation but can occur in advanced disease if bronchial or pulmonary vessels are invaded by tumor.
Pain
There are no pain receptors in the airways or lung parenchyma, so pain generally implies disease extension to the surrounding pleura or mediastinal structures. Another explanation of pain is due to infection distal to an obstructing central tumor. Pleural and mediastinal pain are usually nociceptive and opioid sensitive. Unilateral facial pain due to mediastinal involvement has been described [8]. This is usually felt in or around the ear and is thought to be referred pain via the vagus nerve. It improves with palliative radiotherapy to the tumor. If a peripheral cancer invades the chest wall, it will cause nociceptive pain due to involvement of the pleura, muscle, ribs or connective tissues, and neuropathic pain in the distribution of any intercostal nerve involved. At the apex of the lung, superior sulcus cancers can either invade the brachial plexus (and cause neuropathic pain) or the subclavian sympathetic plexus. The latter is characterised by sympathetically maintained pain of the upper limb and/or an ipsilateral Horner’s syndrome of ptosis, meiosis, enophthalmos and anhydrosis [9].
Metastatic Symptoms
Lung cancer can metastasize via the blood stream to almost any organ. In practice the commonest sites for symptomatic metastatic spread are the brain, bones, liver, pleura, skin, and subcutaneous tissue. The lungs and adrenals are commonly also involved but tend not to give rise to new symptoms. Bone metastases present with bone pain, pathological fracture or nerve compression symptoms such as spinal cord compression. Brain metastases can present with focal neurological symptoms due to the presence of a space occupying lesion, with an epileptic fit, with symptoms of raised intra-cranial pressure or with personality change due to frontal lobe involvement. Liver metastases are characterized by pain due to stretching of the liver capsule or jaundice if the bile ducts are compressed. Pleural involvement results in breathlessness due to effusion or pleuritic pain.
Non-metastatic Symptoms
Also known as para-neoplastic symptoms, these are thought to be caused by secretion of tumor products that act at sites separate from the tumor [10]. Some symptom complexes are not confined to lung cancer, or indeed cancer in general, but occur in a variety of advanced diseases. Fatigue, anorexia, and cachexia are the most obvious examples of this and are found in chronic lung diseases such as COPD, chronic suppurative lung diseases, and idiopathic pulmonary fibrosis (IPF). Secondly there are systemic symptoms that are more commonly associated with lung cancer and mesothelioma. These include sweats and generalized itching (without biochemical abnormality). Clubbing and its rarer companion hypertrophic pulmonary osteo-arthropathy occur in lung cancer but also in chronic lung suppuration, IPF, and cyanotic congenital heart disease. The para-neoplastic syndromes variably associated with lung cancer are listed in Table6.2. Some, such as hypercalcemia, associated with squamous lung cancer, and hyponatremia, associated with small cell lung cancer, are relatively common and their management will be described. Others are much rarer and more exotic. They are more difficult to manage and may not improve even with successful treatment of the primary cancer.
Table 6.2
Summary of paraneoplastic syndromes in patients with lung cancer
Neurological |
Peripheral neuropathy, Lambert-Eaton syndrome, encephalopathy, myelopathy, cerebellar degeneration, psychosis, dementia |
Cutaneous |
Clubbing, dermatomyositis, acanthosis nigricans, pruritus, sweating, erythema multiforme, hyperpigmentation, urticaria, scleroderma |
Musculoskeletal |
Hypertrophic pulmonary osteo-arthropathy, polymyositis, myopathy, osteomalacia |
Blood disorders |
Thrombocytosis, polycythemia, hemolytic anemia, red cell aplasia, dysproteinemia, leukemoid reaction, eosinophilia, thrombocytopenic purpura, hypercoagulable states |
Endocrine |
Cushings, syndrome of inappropriate antidiuretic hormone (SIADH), hypercalcemia, carcinoid syndrome, hyper- and hypo-glycemia, gynecomastia, galactorrhea, growth hormone excess, calcitonin secretion, thyroid stimulating hormone |
Vascular |
Thrombophlebitis, arterial thrombosis, non-bacterial thrombotic endocarditis |
Miscellaneous |
Fatigue, anorexia/cachexia, nephrotic syndrome, hyperuricemia |
Palliative Treatments
Chemotherapy
The value of chemotherapy in prolonging life in lung cancer patients was first demonstrated unequivocally in 1969 in a classic study of 2,000 patients at the Veteran’s Administration Hospitals [11]. It was readily apparent even in those early days that the response rate was vastly superior in cases of small cell cancer. At that time surgery was regarded as the standard treatment of choice for all lung cancer, but in 1973 Matthews and colleagues published their experience of patients who had died within a month of surgical resection of a small cell tumor and showed that systemic metastases were present even though there had been no sign of these at preoperative evaluation [12]. In the following years it became clear that small cell tumors responded to a variety of drugs and that using these in combination produced better results than when these drugs were used as single agents alone [13]. Thus chemotherapy became established as the treatment of choice for small cell cancer.
The obvious problem with chemotherapy is the toxic effects which are both frequent and potentially serious. Even in cases of small cell cancer there has been some debate as to whether the incidence of adverse effects outweighed the survival benefit, particularly in extensive stage disease. When considering the use of chemotherapy in non small cell lung cancer, this issue becomes central. The response rate is clearly less than in small cell cancer and for years many physicians were reluctant to subject their patients to this treatment. Attitudes have changed as new drugs have emerged showing better response rates and less adverse effects. We have now reached a stage where it is appropriate to talk not just about survival benefit from chemotherapy but also about the potential benefits of chemotherapy in terms of overall quality of life.
Small Cell Lung Cancer
The concept of formally measuring quality of life was not properly appreciated when survival benefits of chemotherapy for small cell lung cancer were first being demonstrated. The toxic effects of chemotherapy were, of course, readily apparent, most notably the effects of bone marrow suppression producing anemia, neutropenia with the risk of infection, and thrombocytopenia causing bleeding or bruising. The problems of nausea, vomiting, and hair loss were notorious. Nonetheless, patients with limited stage small cell cancer who had a measurable chance of cure with chemotherapy are generally prepared to accept these, although appreciative of any attempts to mitigate the toxicity.
Reducing the Side Effects of Chemotherapy
The problem of marrow suppression is inevitable with aggressive chemotherapy, although the severity varies. The possibility of using granulocyte colony stimulating factor (G-CSF) to improve marrow function during chemotherapy has been explored [14]. In one study, 403 patients were treated with ACE (doxorubicin, cyclophosphamide and etoposide) given 3 weekly in the control group but every 2 weeks with additional G-CSF in the experimental group. Complete response and survival were improved in the intense treatment group, and quality of life was no worse. The benefit of maintaining the hemoglobin level in patients undergoing chemotherapy for small cell cancer has also been studied. Seventy-four patients with small cell cancer received combination chemotherapy with carboplatin and etoposide with or without additional darbepoetin, an erythropoiesis stimulating agent [15]. The aim in the intervention group was to maintain a hemoglobin of 12–13 g/dL. Whilst darbepoetin reduced the need for blood transfusion and improved some elements of the EORTC (European Organisation for Research and Treatment of Cancer) quality of life questionnaire, it did not produce a global improvement. Both erythropoiesis and granulocyte stimulating agents are expensive and this approach has not been subject to cost-effectiveness analysis. They are not used routinely in the UK.
Can Chemotherapy Improve Quality of Life?
Although chemotherapy agents can induce a range of adverse effects, this has to be set against their potential for shrinking or even curing the cancer, and thereby improving the symptoms produced by the malignant disease itself. Even in patients with relatively good performance status and prognosis, baseline symptoms are common. Thatcher and colleagues reported a cohort of 402 patients who were treated with standard chemotherapy regimens (ACE or carboplatin-etoposide) or with more aggressive ICE-V (ifosfamide, carboplatin, etoposide, and vincristine) [16]. At baseline about one-quarter reported their overall quality of life as poor or extremely poor. Chemotherapy produced improvements in both groups. For ICE-V the percentage with poor or extremely poor quality of life fell from 25 to 13 % at 3 months, and to 14 % at 6 months, while for the standard regime the equivalent figures were 24, 16, and 15 %. Moreover, many specific symptoms such as cough, dyspnea, and appetite improved after chemotherapy was started, although others such as general fatigue and levels of energy did not.
In a later study, Quoix measured quality of life using the functional assessment of cancer therapy-lung questionnaire (FACT-L), and also looked at individual symptoms. This was linked to a comparison of topotecan used with either cisplatin or etoposide. There was no difference in outcome between the two treatment groups, but it was noteworthy that all baseline symptoms improved with both treatment combinations, with the exception of hemoptysis [17].
The balance between prolongation of life and changes in the quality of life is perhaps most important in those patients with poor prognosis. Frustratingly there remains a paucity of good information on quality of life changes with chemotherapy in this group. A Cochrane review found only two studies of first line chemotherapy versus best supportive care in extensive stage small cell lung cancer, comprising a total of 65 patients [18]. The review concluded that the impact of chemotherapy on quality of life in poor prognosis small cell cancer is uncertain. In addition, a large study in which patients with small cell cancer of various stages were randomized to receive either paclitaxel, carboplatin, and etoposide, or vincristine, carboplatin, and etoposide, reported the results separately for the stage four patients [19]. The global quality of life parameters of the EORTC questionnaire improved. However, it is important to note that in this study patients were well enough to be randomized to receive potentially toxic systemic chemotherapy, and thus are not truly representative of the generality of patients with extensive stage disease. It remains the case that the patients with most symptoms (i.e., who have most to gain from treatment) are also those least likely to be able to tolerate chemotherapy.
Non-small Cell Cancer
The role of chemotherapy in non-small cell lung cancer has taken much longer to establish than for small cell malignancy. However, it is now accepted that in patients with a good performance status, chemotherapy confers a survival benefit. Given that the response rate is so much poorer one might have expected studies to have focused more intensely on the balance between extension and quality of life but, as with small cell cancer, formal measurement of quality of life has not been a common feature of studies until fairly recently.
Where possible, non-small cell lung cancer is treated by surgery or radical radiotherapy. There are some key questions to consider about the role of chemotherapy:
What is the value of chemotherapy as a standalone treatment for reducing symptoms of lung cancer?
Does chemotherapy confer any quality of life benefit if used in addition to surgery?
Does chemotherapy confer any quality of life benefits if used in addition to radical radiotherapy?
Patients who are not suitable for surgery or radiotherapy are offered appropriate symptomatic treatment, but they may also wish to try chemotherapy rather than make no attempt to reduce the tumor burden. Among those who are fit enough for chemotherapy there is no doubt that this improves survival. Meta-analysis shows a significantly reduced hazard ratio for death, equivalent to a relative increase in survival of 23 %. In absolute terms this represents an increase in median survival from 4.5 to 6 months [20]. Although this meta-analysis included 16 randomized controlled trials with 2,714 patients, there were insufficient data to formally assess effects on quality of life. Individual controlled trials have reported either improvement or no difference in quality of life in those randomized to chemotherapy compared to those given best supportive care [21–24]. However, it is disappointing that there is no clear evidence of symptomatic benefit given the modest survival benefit.
There has been considerable interest in the use of chemotherapy combined with surgery to improve survival in NSCLC. The earliest trials which used alkylating agents tended to show an adverse affect from the addition of chemotherapy, but more modern studies have suggested that this adjuvant therapy is beneficial whether the chemotherapy is given after surgery or pre-operatively [24]. Details of which sub-groups will benefit are being refined, based on tumor stage. However, once again the benefit in terms of quality of life has not been well documented.
Chemotherapy can also be used in combination with radiotherapy as primary treatment for NSCLC. The chemotherapy can be given at the same time as the radiotherapy (concurrent chemo-radiotherapy) or one can follow the other (sequential chemo-radiotherapy). Both approaches have been studied extensively although with different drug combinations. Concurrent chemo-radiotherapy has been shown to be superior to radiotherapy alone in terms of overall survival and superior to sequential chemo-radiotherapy [25]. However, for both comparisons the concurrent chemo-radiotherapy also caused more symptoms, particularly esophagitis. Global measures of quality of life are lacking.
The assessment of the benefits of chemotherapy is further complicated by the array of drug combinations which have been used in various studies. A comparison of different regimens is beyond the scope of this book, but from the point of view of symptom palliation it is worth highlighting the tyrosine-kinase inhibitors, erlotinib and gefitinib, which act on epidermal growth factor receptors and have been shown to reduce the growth of some types of non-small cell lung cancer. They are not associated with the traditional adverse effects of systemic chemotherapy, although there is a significant instance of skin rashes which can be debilitating. They appear to represent an improvement in terms of the effects on quality of life in patients with NSCLC [26,27].
Radiotherapy
Radiotherapy is a valuable treatment which has been in use in various forms for around 100 years. The development of linear particle accelerators began 60–70 years ago and it has become possible to link this to computed tomography (CT) imaging to deliver the radiation in three-dimensions, further enhancing the precision of therapy. This evolution continues with the advent of stereotactic radiotherapy which essentially aims to deliver radiotherapy more precisely and thus, hopefully, reduce the adverse effects which arise from normal structures being affected by the radiotherapy beam. Although important for the future, this section will concentrate on the current standard forms of radiotherapy.
Radiotherapy can be employed in a variety of ways. It can be used in an attempt to cure a lung cancer, when a high dose is employed and the patient accepts the increased risk of adverse effects that the greater dose entails. Alternatively, lower doses can be used as palliative radiotherapy with a view to reducing tumor bulk and prolonging life but accepting that cure is not likely to be achieved and that the principal value of shrinking the tumor is to improve symptoms. Sometimes there is a particular symptom which the radiotherapy is intended to resolve, and indeed this may not necessarily be in the lung, for example, bone pain due to metastasis from a lung primary is often treated with radiotherapy. Finally, there are some other specific situations in which radiotherapy has been used, for example, cranial irradiation to prevent cerebral metastases after chemotherapy for small cell lung cancer. Each of these situations will be covered in turn.
Radical Radiotherapy
Radical radiotherapy is usually given to patients who are not suitable for surgery because of comorbidity or poor respiratory function, or because they decline surgery. Dosing schedules vary, and this affects toxicity. A meta-analysis of 26 studies showed a wide variation in success rates with 5 year survival figures between 0 and 42 % [28]. As with chemotherapy, the benefits in terms of improvement of quality of life are hard to assess because of a paucity of data. One study which specifically aimed to look at this aspect of radical radiotherapy provided data from 164 patients who received 60 Gy with curative intent, and recorded quality of life using the EORTC questionnaire before treatment and then five times post-treatment up to a 12 month time point. The response rate for improvement in global quality of life was 36 % [29].
Palliative Radiotherapy
It is harder still to synthesize the symptomatic benefit from trials of palliative radiotherapy since the variation in treatment regimes is even greater, because it is not clear in some trials how bad symptoms were before treatment, because of variation in other patient characteristics, and because of variation in outcome measures [30]. One interesting UK trial looked at patients with minimal thoracic symptoms who were unsuitable for resection or radical radiotherapy. These patients were randomized to receive radiotherapy to their thoracic tumor immediately, or to wait until specific symptoms developed. Two hundred and thirty patients were randomized of whom 42 % in the delayed treatment group eventually received radiotherapy after a median wait of 125 days [31]. No difference was seen in symptoms, psychological distress, activity level or survival, implying that treatment can wait until there is at last one specific symptom needing to be addressed.
Palliation of Specific Symptoms
Cough
An unpleasant persistent cough is a common problem in advanced lung cancer particularly with more central lesions as cough receptors are present in greater density in central airways. Radiotherapy is a potential treatment option, the benefit presumably relating to shrinkage of the tumor. The success rate for significant palliation of cough is a little over 50 %, although this estimate is based on retrospective review [32]. One prospective evaluation using a standardized questionnaire found a disappointing response rate of 31 % [29]. The technique of endobronchial radiotherapy (brachytherapy) has been described in Chap.3. It is probably inferior to external beam radiotherapy for palliation of symptoms, but has the advantage of being potentially applicable in patients who have already had maximal dose of conventional radiotherapy, the limiting factor being that the lesion has to be accessible to the bronchoscope in order to position the treatment catheters. Brachytherapy has been used to treat patients with symptomatic tumor recurrence after primary treatment with high-dose radiotherapy and a surprisingly good response rate of 77 % was seen for improvement of cough [33].
Hemoptysis
As with cough, hemoptysis secondary to lung cancer has long been treated with radiotherapy, and brachytherapy is again an option in those patients who have already received external beam therapy. Hemoptysis seems to respond well to radiotherapy. A prospective study using a validating questionnaire showed that the symptom disappeared or improved significantly in 83 % of patients [29]. In a separate retrospective study, the response to brachytherapy as second line treatment of hemoptysis was 92 % [33].
Breathlessness
Breathlessness can arise for several different reasons in lung cancer. When it is caused by the development of a pleural or pericardial effusion, or by anemia, radiotherapy has no role, nor is it of any benefit when dyspnea is caused by lymphangitis carcinomatosa. However, when breathlessness has arisen because of mechanical obstruction of a large bronchus, with or without collapse of the distal lung, radiotherapy may be of value in shrinking the tumor and relieving the obstruction. Unfortunately, even if the tumor responds, re-expansion of collapsed lung is not guaranteed. Experience suggests that expansion is less likely the longer the lung remains in its non-aerated state, and treatment of dyspnea due to obstruction of tumor should consider the role of radiotherapy in conjunction with other treatment such as stents or laser therapy. Because tumor response in this situation does not necessarily lead to lung re-expansion, the response rates for breathlessness treated by radiotherapy tend to be poorer than those for symptoms such as hemoptysis. In a prospective study, the response rate for dyspnea was 37 % [29].
Pain
Lung cancer can produce pain by local invasion of the chest wall or through distant metastases, particularly to bone. The degree of discomfort and the likelihood of a worthwhile response to radiotherapy differ a little with the extent of the invasion, but some relief is usually achieved. A response rate of 68 % has been found in a prospective study [29]. For distant bone metastases, studies have shown that a single fraction of 8 Gy is as effective as higher dose multi-fraction therapy in relieving pain [34]. Response to radiotherapy is usually reasonably quick and treatment need not be withheld because a patient is judged to be within a few weeks of death – improvement of pain at 1 month is found in 70 % of patients [35]. Treatment of bone metastases can be associated with further weakening of the bone, and this is particularly important in weight-bearing sites, such as the femur. Adequate palliation should involve orthopaedic advice, and it may be necessary for patients to avoid weight bearing for a period of time or to undergo a surgical stabilization procedure.
Cerebral Metastasis
The brain is a relatively common site of metastases for both small cell and non-small cell cancer. In the latter case, the metastasis may be solitary and in that situation treatment via surgery or stereotactic radiotherapy with a view to cure may occasionally be appropriate.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


