Lung cancer TNM staging
Primary tumour (T)
T1
Tumour ≤3 cm diameter, surrounded by lung or visceral pleura, without invasion more proximal than lobar bronchus
T1a
Tumour ≤2 cm in diameter
T1b
Tumour >2 cm but ≤3 cm in diameter
T2
Tumour >3 cm but ≤7 cm, or tumour with any of the following features:
– Involves main bronchus, ≥2 cm distal to carina
– Invades visceral pleura
– Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung
T2a
Tumour >3 cm but ≤5 cm
T2b
Tumour >5 cm but ≤7 cm
T3
Tumour >7 cm or any of the following:
– Directly invades any of the following: chest wall, diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium, main bronchus <2 cm from carina (without involvement of carina)
– Atelectasis or obstructive pneumonitis of the entire lung
– Separate tumour nodules in the same lobe
T4
Tumour of any size that invades the mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina, or with separate tumour nodules in a different ipsilateral lobe
Regional lymph nodes (N)
N0
No regional lymph node metastases
N1
Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension
N2
Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s)
N3
Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s)
Distant metastasis (M)
M0
No distant metastasis
M1
Distant metastasis
M1a
Separate tumour nodule(s) in a contralateral lobe; tumour with pleural nodules or malignant pleural or pericardial effusion
M1b
Distant metastasis (in extra-thoracic organs)
Stage
T
N
M
IA
T1a, T1b
N0
M0
IB
T2a
N0
M0
IIA
T1,T2a
N1
M0
T2b
N0
M0
IIB
T2b
N1
M0
T3
N0
M0
IIIA
T1, T2
N2
M0
T3
N1,N2
M0
T4
N0,N1
M0
IIIB
T4
N2
M0
Any T
N3
M0
IV
Any T
Any N
M1
Laboratory tests (CBC, serum chemistries, renal function tests, LDH, liver function tests)
Pulmonary Function Tests (PFTs)
Radiographic Evaluation
Chest X-Ray
First-line modality, usually followed by CT for detailed evaluation.
Lesions at a minimum of 7–10 mm in diameter can be visualised.
Assess for number and sites of lesion (central/peripheral), secondary effects of tumour (consolidation/atelectasis due to segmental or lobar collapse), presence of effusion, and advanced bone lesions.
CT Chest (with IV contrast)
Used to evaluate size, location, number of lung lesions, features of malignancy (scalloped borders, spiculations, corona radiata, lack of calcifications, ground glass opacities [5]), tumour cavitation and necrosis, secondary effects (including atelectasis, post-obstructive pneumonia, pleural effusion), extent of invasion into adjacent structures, chest wall or mediastinum, and mediastinal lymphadenopathy.
Lymph node metastasis sensitivity: 51 %; specificity: 86 % [6]
Lymph nodes between 10 and 15 mm have a 50 % risk of malignant involvement, while those >15 mm have a 67 % risk.
Abdominal cuts also included to look for distant metastases (e.g. adrenals and liver).
Brain CT (with IV contrast) should be performed if MRI is not available to rule out metastases in selected patients.
PET-CT
Helps to identify diseased nodes in normal sized lymph nodes on CT [7], distinguish benign and malignant pulmonary nodules and other sites with remote metastasis.
Minimal role in patients with obvious metastatic disease.
A systematic review on the diagnostic properties of PET-CT for mediastinal lymph node metastasis demonstrated a sensitivity 83 %, specificity 96 %, and accuracy >90 % [6, 8].
Although it has high sensitivity (96 %) for discrimination of malignant from benign nodules, it has lower specificity (78 %) and a positive-predictive value of 91 % due to false positives secondary to increased uptake in inflammatory, granulomatous, or infectious conditions [8, 9].
Some tumours (i.e. typical carcinoids) tend to have low metabolic activity and are not be PET-avid.
If any uncertainty exists, confirmation of positive findings should be made with tissue diagnosis before ruling out pulmonary resection for lung cancer.
MRI
Used for tumour delineation with suspected local invasion into brachial plexus, superior sulcus vessels, vertebral body or spinal cord, and for brain metastases. Otherwise it does not offer significant advantages over CT.
MRI is the gold standard for evaluating the brain for any evidence of metastases in selected patients.
Invasive Staging (Non-Surgical Tissue Diagnosis):
Percutaneous transthoracic needle aspiration (TTNA)
For peripherally located lesions >1 cm (lacks accuracy for subcentimetric lesions)
TTNA allows the clinician to secure a tissue diagnosis preoperatively, thereby avoiding potentially unnecessary pulmonary resection if a benign diagnosis is made (e.g. necrotizing granuloma).
For new, growing and resectable nodules, TTNA may not change management.
Flexible bronchoscopy (Fig. 3.1)
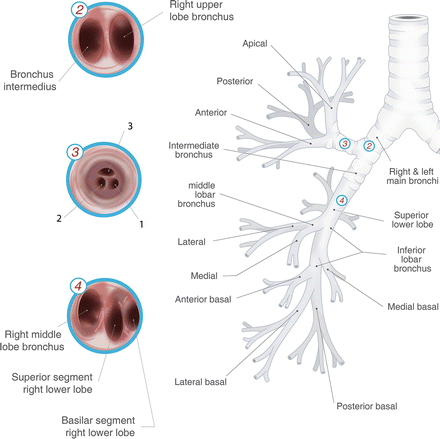
Fig. 3.1.
Tracheobronchial tree with intra-luminal bronchoscopic view. Used with permission from the McGill University Health Centre Patient Education Office.
Includes forceps biopsy, brushings, saline lavage, transbronchial needle aspiration (TBNA).
Can be used to sample centrally located primary tumours and lymph nodes.
Adjunct guidance improved with fluoroscopy for TBNA.
Endobronchial ultrasound (EBUS) guided TBNA
Transesophageal endoscopic ultrasound guided FNA (EUS-FNA)
Especially useful for subcarinal, aortopulmonary, paraesophageal and pulmonary ligament lymph nodes. Sensitivity: 92 %; specificity: 100 %; accuracy: 97 % [15].
Used in conjunction with EBUS to sample all lymph node stations.
EBUS-FNA: anterior and superior lymph nodes
EUS-FNA: posterior and inferior lymph nodes
Can also be used to characterise the primary tumour’s extent of invasion.
Invasive Staging (Surgical Tissue Diagnosis):
Cervical mediastinoscopy
Can be done using direct optic visualisation or video-assisted mediastinoscopy with sensitivity and specificity of 78 % and 100 % respectively, and 11 % false-negative rate [16].
Samples upper and lower paratracheal lymph nodes above the aortic arch.
Access to anterior subcarinal and bilateral hilar nodes are technically challenging.
Not a necessary or routine step in staging (can be eliminated in clinical T1aN0 disease—namely, tumours that are <2 cm in maximal diameter, with negative PET-CT).
Chamberlain’s procedure (anterior mediastinotomy)
Access through the second or third intercostal space to left paratracheal, para-aortic, subaortic and subcarinal nodes.
Video-Assisted Thoracic Surgery (VATS)
Can provide biopsies of paratracheal, azygos, paraesophageal, pulmonary ligament, subaortic and para-aortic lymph nodes.
Management—Non-Small Cell Lung Carcinoma [19]:
Locoregional Disease (Fig. 3.2):
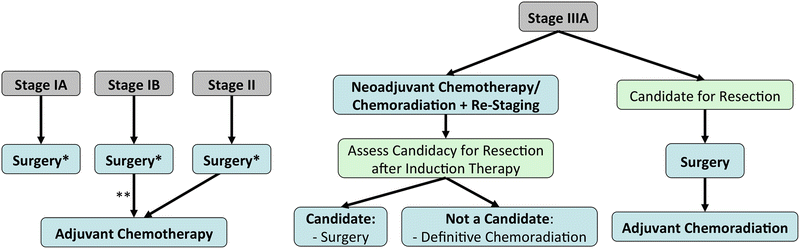
Fig. 3.2.
Management algorithm for locoregional and locally advanced non-small-cell lung cancer. *: R1 resections should undergo either re-resection (with or without chemotherapy) or chemoradiation. **: High-risk features for stage IB NSCLC (e.g. >4 cm) should be considered for adjuvant chemotherapy.
Surgical resection is standard-of-care for localised disease.
Most patients with stage I disease following an R0 resection do not require adjuvant chemotherapy.
Meta-analysis of cisplatin-based chemotherapy in patients with resected stage 1 NSCLC showed no survival advantage (stage 1A: HR 1.40, 95%CI 0.95–2.06; stage 1B: HR 0.93, 95%CI 0.78–1.10) [20–22].
However, CALGB-9633 study suggested that adjuvant chemotherapy has a significant survival advantage in stage IB patients with tumour size >4 cm (HR 0.69, 95%CI 0.48–0.99) [23].
Several meta-analysis and randomised controlled trials suggest a significant survival advantage for stage II patients receiving adjuvant chemotherapy (HR 0.83, 95%CI 0.73–0.95) [20, 21, 24].
Adjuvant radiation therapy does not improve outcomes of patients with stage I disease following an R0 resection [25].
Locally Advanced Disease (Fig. 3.2):
Although induction therapy for stage III disease improves survival, the choice of subsequent locoregional treatment is debated.
Neoadjuvant chemoradiation compared to neoadjuvant chemotherapy increases the pathological response rate (60–65 % vs. 20–35 %) and mediastinal node downstaging (46 % vs. 29 %, p = 0.02) [26, 27]. However there is no difference in progression-free survival or overall survival.
Stage IIIA Disease (T3N1, T4N0-1):
Complex cases should undergo discussion at pulmonary oncology multidisciplinary rounds.
Patients should be assessed for resectability and the probability of achieving an R0 resection.
If unresectable, concurrent chemoradiation is the standard of treatment.
If deemed resectable, options include upfront resection followed by chemoradiation; or preoperative chemoradiation followed by surgery, with or without re-staging of the mediastinum, followed by adjuvant chemotherapy.
Certain lesions that are invading adjacent structures may benefit from aggressive en-bloc resections (including the vertebrae, carina, atrium and chest wall).
Patients with an R1 resection or unresectable disease should undergo chemoradiation.
Stage IIIA Disease with N2 (T1-3):
Complex cases should undergo discussion at pulmonary oncology multidisciplinary rounds.
Management of locally advanced N2 disease remains controversial [28]. However it seems that tri-modality therapy (induction chemoradiation followed by surgery) compared to definitive chemoradiation without surgery, improves progression-free survival but not overall survival, except for the subset of patients undergoing lobectomy, and not pneumonectomy [29].
Definite or induction chemoradiation followed by re-assessment for disease progression can guide surgical management. If there is no progression, surgery followed by adjuvant chemotherapy is an option. If there is disease progression, chemoradiation or chemotherapy for local or systemic control should be considered.
Advanced Disease (Stage IIIB and IV):
Chemoradiation offers a survival advantage
Palliative care to control morbidity from advanced disease (i.e. pleurodesis or long-term catheter drainage of malignant effusions, transbronchial stenting)
Targeted systemic therapy for distant metastases
Surgical Principles (Fig. 3.3):
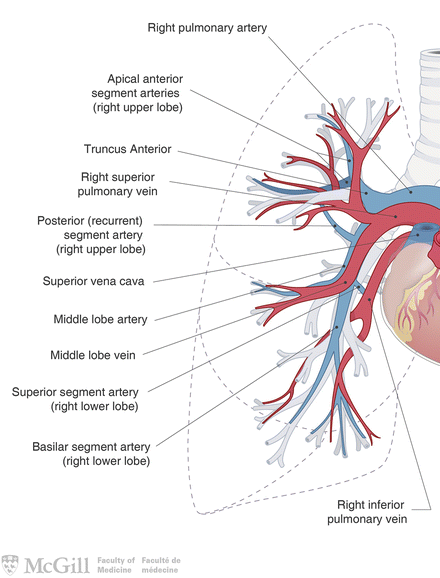
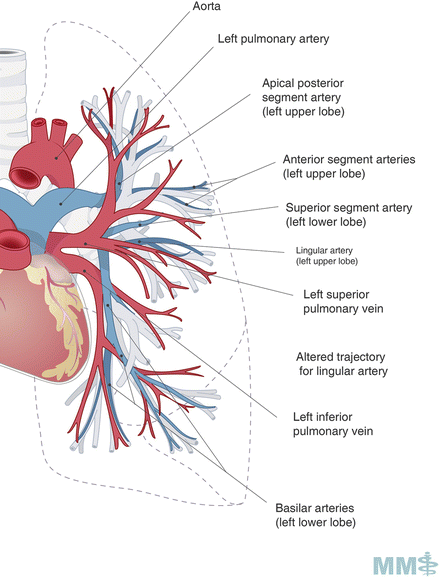
Fig. 3.3.
Pulmonary arteries and veins. Used with permission from the McGill University Health Centre Patient Education Office.
Anatomic resection in order to remove cancer and adjacent lymph nodes: lobectomy (most common), pneumonectomy, bilobectomy, sleeve lobectomy or segmentectomy. Lobectomy is considered standard-of-care for stage I NSCLC in patients with adequate pulmonary reserve.
Non-anatomic resection (if risk of lymph node involvement is exceptionally low): wedge resection.
Minimally invasive approach (VATS):
VATS lobectomy shown to decrease blood loss, chest tube drainage time, hospital length-of-stay, post-operative pain, and perioperative complications, while achieving improved oncologic outcomes compared an open approach (5-year survival rate OR 1.82, 95%CI 1.43–2.31) [34].
Relative contraindications: significant mediastinal lymphadenopathy, tumours >5 cm and centrally located tumours.
Never a contraindication for initiating an elective pulmonary oncologic resection thoracoscopically.
Extent of lymph node dissection should include both N1 and N2 stations (minimum of 3 N2 stations).
Sampling multi-station lymph nodes is essential for accurate staging, to allow patients to benefit from adjuvant therapy (Fig. 3.4). Although resection of lymph nodes has never been proven superior to sampling, it is good surgical practice to resect lymph nodes when possible, without incurring increased risk of harm to the patient from over-zealous and unnecessary lymph node dissection.
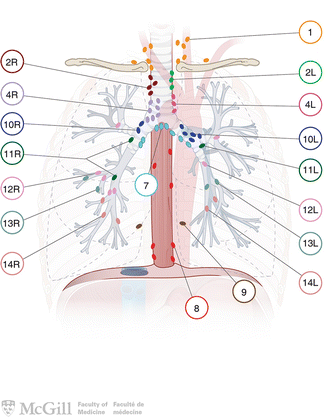
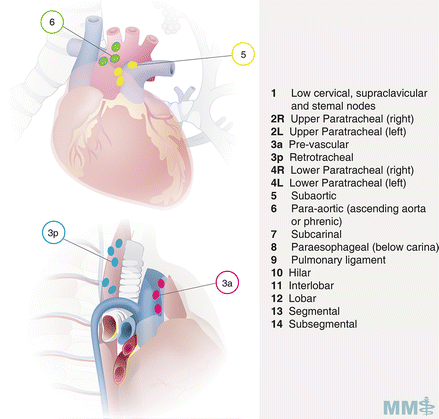
Fig. 3.4.
Lymph node classification map [35]. Used with permission from the McGill University Health Centre Patient Education Office.
Medically unfit patients should be considered for lesser resection (segmentectomy or non-anatomic wedge resection), definitive radiotherapy, chemoradiation or stereotactic radiation therapy.
Pathologic Evaluation
Prognostic and Predictive Biomarkers:
Epidermal growth factor receptor (EGFR)—predictive of response to EGFR-TKI therapy
ERCC1 (improved survival and poor response to platinum chemotherapy)
KRAS oncogene (decreased survival and resistance to TKI therapy)
Anaplastic lymphoma kinase (ALK) fusion oncogene (prognostic of resistance to EGFR TKI therapy)
Recurrence
Locoregional Recurrence:
Endobronchial obstruction: radiation therapy, photodynamic therapy, laser, stents, surgery
Resectable lung recurrence: surgery, radiation therapy
Mediastinal lymph node recurrence: chemoradiation, systemic chemotherapy
SVC obstruction: chemoradiation, radiation therapy, SVC stent
Severe haemoptysis: radiation therapy, brachytherapy, laser, photodynamic therapy, angioembolization, surgery (endobronchial, VATS or open). See Chap. 3 : Lung and Airways (Haemoptysis).
Distant Metastases
Diffuse brain metastases: palliative radiation therapy
Bone metastases: palliative radiation therapy, stabilisation (if at risk of fracture), bisphosphonate therapy
Disseminated metastases—testing for EGFR, ALK with subsequent targeted therapy
Management—Small Cell Lung Carcinoma (Fig. 3.5)
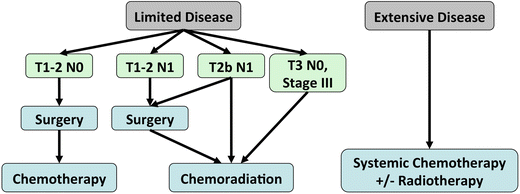

Fig. 3.5.
Management algorithm for small-cell lung cancer.
>50 % of patients present with advanced, disseminated disease and are ineligible for surgery.
A 2-stage system has been used to classify SCLC, based on the ability to include all disease within the field for external-beam radiation therapy:
Limited disease
Extensive disease
Limited Disease (corresponding to stage I-IIIB): tumour confined to one hemithorax, regional nodes (ipsilateral and contralateral hilar and mediastinal), and ipsilateral supraclavicular nodes.
Extensive Disease (corresponding to stage IV): tumour has spread beyond the boundaries of limited disease (distant metastasis, malignant pleural or pericardial effusions, contralateral supraclavicular nodes).
Highly sensitive to systemic chemotherapy but with a high rate of relapse [38].
Objective response rate: 60–80 %
Complete response: 25–50 % of patients with limited disease
Platinum-based combinations are often the initial first line chemotherapy regimens with multiple other combinations used as alternative and second-line regimens.
Thoracic Radiation therapy
Surgery
Unlike NSCLC, lung resection plays a limited role in the multimodality management of SCLC with no improvement in survival, even for limited stage SCLC [41].
However, for very early SCLC, surgery followed by adjuvant platinum-based chemotherapy has been shown to have 5-year survivals as high as 86 % (stage I) and 49 % (stage I and II) and a median overall survival of 47 months (stage I and II) [42, 43].
For patients discovered to have node-positive disease on post-operative pathology, adjuvant therapy should also consist of radiation therapy.
Prophylactic cranial irradiation
Lung Metastases from Other Primary Tumours
Overview
Lung is a common location for distant metastasis from other primary neoplasms.
Biologic behaviour of the underlying disease will predict the mechanism of dissemination, pattern of metastasis and aggressiveness.
Although new pulmonary lesions in a patient with a known primary tumour are highly indicative of metastases (especially where they are multiple), they can also be incidental findings of benign lung lesions or a new metachronous lung cancer (especially when there is a new solitary lung lesion in the absence of any extra-thoracic metastasis) [48].
Surgical Metastasectomy
Given that the prognosis of patients with metastases to the lungs is highly heterogeneous, pulmonary resection may improve long-term survival for a subset of patients.
For a select group of patients whose primary tumour is under control, pulmonary metastasectomy is feasible and safe.
Clear communication, possibly including multidisciplinary oncology rounds discussion, with the referring oncologist regarding strategy of resection and systemic therapy, should be undertaken in all patients.
Several factors can predict a favourable prognosis post-metastasectomy [49, 50]:
Resectability: a complete resection has been shown to be an independent prognostic factor.
Patients should be medically fit with adequate pulmonary reserve to tolerate a resection.
Disease-Free Interval: a longer disease-free interval (>36 months) is associated with less aggressive behaviour and a greater likelihood of cure following lung resection.
Histopathology: colon cancers, germ cell tumours, sarcomas, breast cancers and well-differentiated thyroid cancers have shown long-term benefit [51–54]. Even certain tumours that are considered to have an aggressive disease course (such as esophageal cancer) have demonstrated a survival advantage in select cases [55, 56].
Number of Metastatic Lesions: patients with more than 1 nodule have decreased survival; however there is no absolute number of metastases that differentiate between surgical and medical disease. With favourable resectability, disease free interval, histopathology and cardiopulmonary fitness, multiple and bilateral metastases may certainly be resected.
Low Tumour Burden
All aforementioned factors should be taken into consideration by a multidisciplinary tumour board to decide if a patient is a surgical candidate.
Disseminated disease is typically considered a contraindication, although certain cases of colon cancer with lung and liver metastases are amenable to metastasectomy.
Non-anatomic wedge resections are typically performed due to the high risk of other pulmonary recurrent metastatic lesions and the need for subsequent resections, as well as the lower risk of lymph node recurrence due to secondary metastatic pulmonary tumours. Albeit rare, the presence of lymph node metastases in the mediastinum due to secondary pulmonary neoplastic disease is generally considered a contraindication to resection due to poor prognosis.
Section 2: Tracheal Disorders
Tracheal Cancer
Epidemiology and Histopathology
Clinical Presentation and Workup
Obstructive symptoms: chronic cough, dyspnea (especially after exertion), stridor, postural-wheezing, post-obstructive pneumonia, respiratory failure.
Haemoptysis
Symptoms of local invasion: hoarseness
Often patients are mis-diagnosed as having adult-onset asthma or COPD, especially with a history of smoking.
All patients should undergo CT neck/chest/abdomen, bronchoscopy and esophagogastroscopy for detailed characterization and tissue diagnosis.
Management (Fig. 3.6)
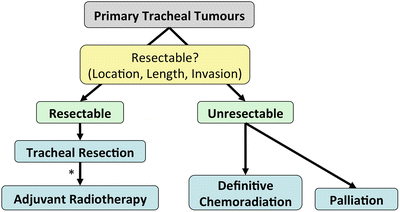

Fig. 3.6.
Management algorithm for primary tracheal tumours. *Patients with R1 or R2 resections or squamous-cell histopathology should undergo adjuvant radiotherapy.
Since the best chance for cure is tracheal resection with or without adjuvant radiotherapy, as opposed to definitive chemoradiation [57], patients should undergo evaluation to determine if their tumour is resectable.
Resectability is determined by tumour location, length and invasion of adjacent structures.
The benefits of performing an oncologically sound en-bloc resection with negative margins should be weighed against the risk of dehiscence, which has high rates of mortality.
Radiotherapy is reserved for unresectable tumours as well as adjuvant therapy for most patients, especially R1/R2 resections and squamous-cell carcinomas.
Chemotherapy is reserved for unresectable and end-stage disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


