Absolute indications for amputation
Relative indications for amputation
Nonsalvageable foot or lower extremity due to extensive tissue loss
Severe Charcot foot deformity limiting functional status
Nonsalvageable foot or lower extremity in the setting of acute limb ischemia
Failed prior revascularization with continued tissue loss or infection
Severe life-threatening foot/limb infection
Poor surgical candidate with critical limb ischemia
Nonambulatory patients with nonhealing wounds or infection including patients with severe lower extremity contractures
No revascularization targets in patients with critical limb ischemia and ongoing tissue loss or severe rest pain
Chronic osteomyelitis and nonhealing wounds
The first decision point along the path to amputation determines whether the lower extremity is worth saving. Nonsalvageable lower extremities suffer from extensive tissue loss that would be impossible to heal or would require massive debridement ultimately compromising the architecture and function of the foot. Other factors favoring a lower extremity amputation include extensive heel gangrene particularly with underlying osteomyelitis, widespread soft tissue infection, and severe Charcot deformity of the foot with loss of function. Any of these clinical scenarios represents a clear indication for lower extremity amputation.
If the patient does not have an obvious indication for amputation because of the factors described above, the next step involves determining the etiology of the lower extremity wounds. Patients may present with ischemic, neuroischemic, neuropathic, pressure-induced, or venous wounds. The investigation begins with a complete pulse exam to evaluate the vascular supply to the lower extremity. Absence of palpable lower extremity pulses frequently indicates underlying peripheral arterial disease (PAD). An ankle-brachial index (ABI) provides an objective, bedside exam to detect PAD defined as an ABI less than 0.9. A systolic ankle pressure less than 70 mmHg identifies patients with severe ischemia of the foot who are unlikely to heal their wounds without a revascularization procedure [6]. Falsely elevated ABIs (greater than 1.3) usually indicate tibial vessel calcification. In this situation, toe pressures measured by the noninvasive vascular lab provide a more accurate assessment of perfusion with values less than 50 mmHg indicating significant foot ischemia. Transcutaneous pressures may also help predict wound healing after an amputation. Several studies have shown that a threshold level of 30 mmHg correlates with improved wound healing. Ballard et al. reported a 73 % healing rate at this level, while Chiriano et al. found that 67 % of patients with PAD and underlying wounds healed with a TcpO2 level above 30 mmHg [10–12].
If ischemia caused the lower extremity wounds, the next step involves determining which patients will benefit from revascularization. Although revascularization is the treatment of choice for patients with underlying arterial disease and tissue loss, it is not universally successful. Lower extremity wounds can recur after failed surgical bypasses or endovascular interventions. Abou-Zamzam et al. found that over 50 % of patients undergoing an amputation had either a prior attempt at revascularization or no revascularization options based on anatomy [13]. In patients with limb-threatening ischemia, the surgeon must determine the feasibility and benefit of revascularization.
Imaging studies such as computed tomography angiography (CTA) or magnetic resonance angiography (MRA) can map the arterial anatomy to help determine revascularization options. Although CTA and MRA can detect iliac, femoral, and popliteal artery lesions, their accuracy diminishes in the tibial vessels. Catheter-directed angiography may be indicated if noninvasive imaging does not identify target vessels for revascularization. Patient factors that make angiography unreasonable include severe renal insufficiency in a highly debilitated patient or progression of wounds that would make the limb unsalvageable. Angiography should include images of the foot to evaluate for distal revascularization targets. While axial flow to the foot has traditionally been preferred for healing pedal wounds, a peroneal artery bypass can be successful if adequate collaterals to the pedal vessels are present at the ankle [14].
Patients who have no revascularization targets usually require an amputation. Some patients have distal arterial targets but lack an autogenous conduit to function as a bypass. Although distal bypasses with prosthetic grafts are feasible, they have inferior patency rates and increased infection risk [15]. Debilitated, nonambulatory patients with extensive ischemic tissue loss and no autogenous conduit are better served with an amputation instead of repeat revascularization.
Patients without underlying peripheral arterial disease who present with nonhealing lower extremity wounds most often suffer from diabetes. Several studies cite diabetes as the most common risk factor for amputation because of its association with infection, neuropathy, and architectural deformity of the foot. Malone ranked the indications for amputation starting with complications of diabetes (60–80 %), infections without diabetes (15–25 %), ischemia without infection (5–10 %), chronic osteomyelitis (3–5 %), trauma (2–5 %), and miscellaneous (5–10 %) [2, 16]. Diabetic patients can present with overwhelming infections which require urgent amputation as a matter of life over limb. Elective amputations may be necessary for loss of function after multiple wound debridements for underlying osteomyelitis or severe Charcot deformity. Preventing amputations in patients with foot deformities or neuropathic ulcers requires orthotics to off load pressure on the foot, advanced wound care, and surgical correction of architectural deformities whenever possible. Fortunately, most patients who undergo an amputation for the indications described above maintain an acceptable quality of life.
Functional status represents the final component to evaluate in patients being considered for amputation. Invasive limb salvage procedures have no role in the management of extremely debilitated patients. Nonambulatory patients with extensive lower extremity wounds and patients with fixed joint contractures and pressure-induced tissue necrosis should undergo primary amputation. Patients with limited survival including patients with malignancy, severe CHF, and severe dementia and elderly patients on dialysis should be considered for primary amputation [17–19].
Determining the Level of Amputation
Although an amputation is often perceived as a failure of limb salvage, it still requires preoperative planning and sound surgical judgment. Choosing the appropriate level of amputation plays a critical role in achieving a successful outcome. Amputations that fail to heal delay rehabilitation and frequently require further surgery with its associated risks. While the amputation level should be tailored to each patient’s anatomic and functional needs, any amputation must adhere to three general principles. Firstly, it should ensure the removal of all nonviable tissue and infection. Secondly, the level of amputation should be chosen to maximize the chances of uncomplicated wound healing. Finally, it should give the patient the best opportunity for future prosthetic use.
Successful healing after amputation depends on multiple factors including hemodynamic status, infection, glucose level, and the patients’ underlying comorbidities (Table 8.2). It should be noted that although wound healing is the easiest outcome variable to observe, it is not the only measure of success after an amputation. Other important outcome measures for amputations include successful rehabilitation, functional status, and overall survival.
Table 8.2
Factors that influence healing at amputation site
SPP | >30 mmHg |
TcPO2 | >30 mmHg |
Ankle pressures | >70 mmHg |
Toe pressure | >50 mmHg |
Physical exam | Palpable popliteal pulse |
Level of amputation | Below the ankle < above the ankle |
Presence of comorbidities | CAD, cerebrovascular disease, ESRD, diabetes, COPD, increased age |
While there is no consensus on which noninvasive tests can determine the optimal level of amputation, it is clear that physical exam alone and presence of bleeding during debridement are poor predictors of successful wound healing. Although the presence of a palpable pulse immediately proximal to the amputation site nearly always predicts healing, the absence of a pulse does not consistently lead to wound failure [2]. Using the pulse exam alone to determine amputation level would therefore unnecessarily preclude some patients from having more distal amputations. Several studies have documented toe pressures and TcPO2 levels as predictors of wound healing particularly in forefoot procedures such as digit, ray, and transmetatarsal amputations. Vitti et al. retrospectively studied 136 men undergoing forefoot amputations and found universal failure of healing in diabetic patients with toe pressures less than 38 mmHg and universal success with toe pressures greater than 68 mmHg. This threshold did not predict outcome in nondiabetic patients [20].
Several studies have explored alternatives to the “clinical judgment” method of determining amputation level which has proven to be notoriously inaccurate. Poredos et al. attempted to establish a minimal TcPO2 level necessary for amputation healing by studying 71 limbs of which 55 were below knee amputations. In all patients, the level of amputation was based solely on clinical factors. Sixteen patients (22.5 %) required conversion to above knee amputation due to failure of healing. The patients that failed to heal had significantly lower TcPO2 levels versus those that healed (18 mmHg vs. 37 mmHg, p < 0.01) [21]. Holstein found that only one failed below knee amputation out of 15 exhibited clinical signs of ischemia prior to amputation, whereas all of the failures had skin perfusion pressures (SPP) less than 30 mmHg. Only 3 % of the amputations in this series with an SPP greater than 30 mmHg failed to heal [22]. These studies and others like them highlight the inaccuracy of clinical judgment alone in deciding on the level of amputation. Skin perfusion pressure seems to correlate well with below knee amputation healing; however, other factors also contribute to successful outcomes following major amputation.
Ankle and toe pressures should be measured as surrogates for local perfusion at the site of the surgical procedure. A study of 44 consecutive major lower extremity amputations (38 for severe limb ischemia) revealed that all patients with ankle pressures greater than 70 mmHg achieved successful wound healing. In contrast only 50 % of those with ankle pressures less than 70 mmHg healed their wounds. Notably, these findings were only significant for amputations proximal to the ankle; foot amputations had worse outcomes overall. The authors found no significant outcome differences with respect to skin temperature, gender, age, blood chemistry, or duration of diabetes [23]. Data from large series suggest that TcPO2 and skin perfusion pressures are also less significant predictors of wound healing in forefoot amputations compared to more proximal amputations [24]. Toe pressures may offer a more accurate predictor of healing after forefoot amputations. The TASC II document cites toe pressures less than 50 mmHg as a sign of severe ischemia at the level of the forefoot suggesting that this may increase the risk of failed healing in forefoot amputations [6].
Although hemodynamic factors play an important role in selecting the level of amputation, other more global variables such as prior ambulatory status and presence of comorbidities may also help the surgeon select which amputation to perform. A retrospective review of 80 patients undergoing 91 transmetatarsal amputations found that initial healing of the amputation negatively correlated with the presence of end-stage renal disease and infection [25]. In a different series of patients, the authors found no correlation between healing transmetatarsal amputations and preoperative demographic factors such as CAD, DM, and renal insufficiency. In this cohort, a history of MI and the presence of COPD were associated with mortality after BKA, while ESRD and COPD were the strongest predictors of mortality after AKA [24]. These comorbid conditions seem to correlate better with amputations proximal to the ankle and lose some degree of predictability for foot and toe amputations.
One of the largest outcome studies of lower extremity amputations used the NSQIP database including 4,250 patients from 121 hospitals nationally. In this study, wound complication risk increased with an elevated INR, age 50–59 as compared with older patients, high BMI, and current tobacco use. Thirty-day mortality was 7.6 and 12 % after BKA and AKA, respectively, and predictors of mortality corroborated previous study results with history of MI and presence of COPD associated with mortality after BKA and ESRD and COPD predicting mortality after AKA [26]. Although predicting outcomes based on preoperative risk factors is not always reliable, it is clear that wound complications increase with uncontrolled diabetes, presence of renal disease, COPD, and CAD. These results highlight the importance of pursuing medical optimization prior to performing an elective amputation.
While preoperative planning focuses on achieving local wound healing, a healed stump does not completely define a successful amputation. The amputation level selected should also maximize the functional capacity of the patient. Achieving this goal requires integrating multiple factors that influence the amputation level including extremity perfusion, comorbid conditions, pre-amputation ambulatory status, and life expectancy. Taylor and associates reported on a comprehensive definition of amputation success in 3,000 patients undergoing major lower extremity amputations (309 BKAs) from 1998 to 2004. They defined success as wound healing without need for revision to a higher level, maintenance of ambulatory status for 1 year, and survival for at least 6 months. Only 51 % of patients met this definition of success and the overall mortality was 3.2 %. Preoperative factors associated with poor outcomes included CAD, cerebrovascular disease, COPD, diabetes, impaired ambulatory status, and increased age. Independent predictors of clinical failure included presence of coronary artery disease, cerebrovascular disease, and impaired preoperative ambulatory status. If any two of these factors were present, the probability of success was only 23 %, and if all were present, the success rate dropped to 10.4 %. The probability of success was 67.5 % if none of the factors were present [27].
Return to ambulation should be a primary consideration when determining amputation level. Ambulating with a prosthetic requires more energy, and the energy expenditure increases as the level of amputation moves proximally. Tang et al. found that below knee amputations, knee disarticulation, above knee amputation, and hip disarticulation have energy requirements above baseline of 10–40 %, 71.5 %, 63 %, and 82 %, respectively [28]. Approximately 80 % of patients return to ambulation after a below knee amputation and up to 50 % after an above knee amputation, and less than 10 % of patients walk after a hip disarticulation.
In summary, determining the level of amputation remains challenging and has a significant clinical impact. Although forefoot and below knee amputations have acceptable functional outcomes, they carry an increased risk of wound complication and need for proximal revision. The overall mortality for amputations ranges from 6 to 17 % and peaks among patients with renal failure, prior coronary revascularization, advanced age, and transfemoral versus transtibial amputation [26, 27, 29–32]. In general, transmetatarsal amputations have a significantly lower healing potential than amputations proximal to the ankle and should be reserved for patients with high functional status who have few if any hemodynamic risk factors for wound failure. Most patients who have failed prior revascularization for critical limb ischemia will not be candidates for amputations below the ankle because of impaired preoperative ambulatory status or severe comorbid conditions including renal dysfunction.
Below knee amputation is a reasonable option for ambulatory patients with hemodynamic factors that predict postoperative wound healing including adequate skin perfusion pressures (generally above 30 mmHg). The presence of a palpable popliteal pulse has also been consistently associated with successful wound healing [2]. An overall successful wound healing rate of 85 % should be expected from patients undergoing below knee amputations. Above knee amputations are indicated for nonambulatory or minimally ambulatory patients and for patients who have a low likelihood of healing any distal amputation. These criteria apply even when the wound is completely confined to the forefoot.
Procedures
Digital Amputation
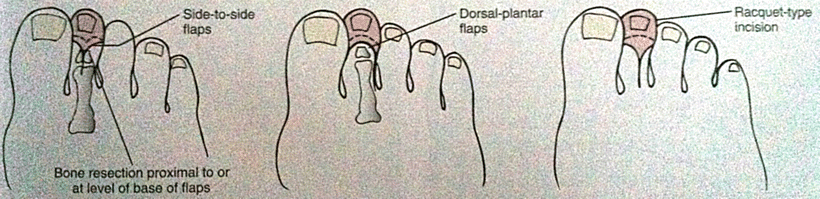
While digital amputations can be performed at several levels, this section will focus on metatarsophalangeal amputations. Planning the skin incision should allow for adequate skin coverage after bony amputation. Usually an elliptical incision oriented in the vertical direction minimizes the space between the digits after wound healing. The incision should begin at the level of the proximal phalanx providing there is viable skin at that level. Dissection should then proceed circumferential through the soft tissue to expose the bone of the proximal phalanx and the metatarsophalangeal joint space. Any bleeding from the digital arteries can usually be controlled with electrocautery. The metatarsophalangeal joint should then be disarticulated. If this is not possible, the proximal phalanx can be transected with a bone cutter and the bone can be removed back to the joint space using a rongeur. We prefer to rongeur the cartilage off of the head of the metatarsal bone because its relative avascularity can complicate wound healing. After achieving hemostasis, closure involves interrupted subcutaneous absorbable sutures followed by interrupted simple nylon sutures for the skin (Fig. 8.1). Amputations performed for acute infection should be left open and packed.
Ray Amputation
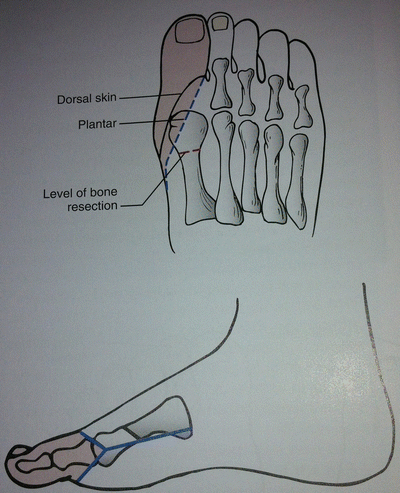
Ray amputation refers to removal of the toe with a part of its corresponding metatarsal bone. The skin incisions vary depending on which toe is being amputated, but all incisions attempt to preserve as much skin as possible for closure. Flap coverage of the remaining bone segment can be achieved with a subperiosteal resection of the metatarsal proximal to the soft tissue incision. For the first and fifth toe resections, the incision is similar to a digital amputation with a proximal extension along the medial (first toe) or lateral (fifth toe) foot to obtain access to the metatarsal bone. The incision is closed along its length after resection of the bone. For central ray resections, the incision again is similar to that of a digital amputation with extension proximally along the dorsal aspect of the foot. Preserving the plantar skin whenever possible plays a critical role in recovery as it will allow for early weight bearing [33] (Fig. 8.2).
Transmetatarsal Amputation (TMA)
Patients being considered for a transmetatarsal amputation should have adequate plantar skin to create a flap that will heal and provide soft tissue support for any forefoot prosthesis. If the plantar skin is compromised, a TMA can be performed in a guillotine fashion with subsequent skin grafting. The incision begins transversely across the dorsum of the foot at the level of the midmetatarsal. It then extends distally on the medial and lateral side of the foot and before crossing on the plantar aspect of the foot at the level of the metatarsophalangeal joint to create a posterior flap. Dissection is performed through the subcutaneous tissue along the incision line. After exposing the metatarsal bones, a periosteal elevator is used to create a cephalad flap on the dorsal surface. The skin should be retracted cephalad and the metatarsal bones should be transected at the level of the midshaft or proximal third. In order to preserve the plantar fat pad, a periosteal elevator is used to separate the plantar surface of the metatarsal bones from the soft tissue. The digital arteries can either be suture ligated or cauterized to obtain hemostasis. Starting with the transected shafts of the metatarsal bones, the fat pad is then dissected away and the forefoot is removed. A tension-free closure is then performed by approximating the fascia of the dorsum of the foot with the soft tissue of the plantar fat pad with interrupted absorbable sutures. The skin can either be stapled or closed with interrupted 3–0 nylon sutures. There is no need for a drain in the subcutaneous tissue; however, a local pump that functions to slowly infuse local anesthetic may help reduce the need for narcotics postoperatively. The foot is wrapped with moderate compression to minimize the risks of postoperative hematoma or edema (Fig. 8.3).
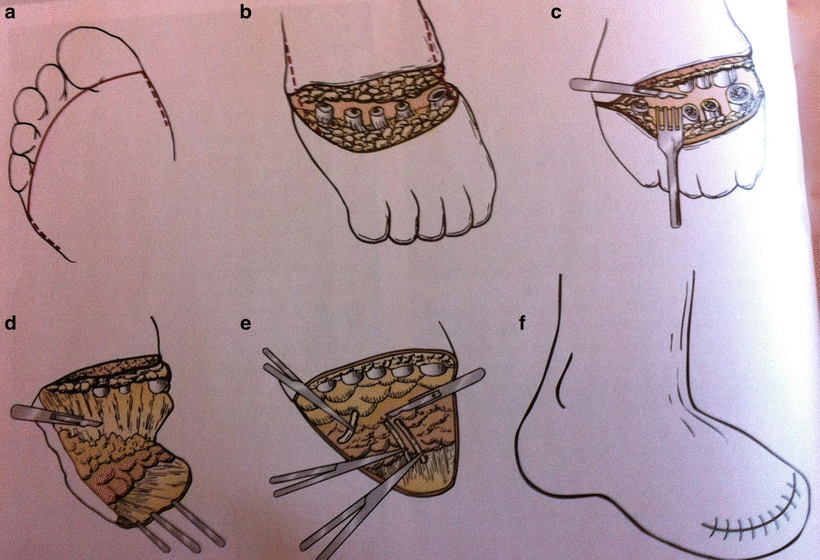

Fig. 8.3




Transmetatarsal amputation. (a) Incision on plantar aspect of foot at level of metatarsophalangeal joint. (b) Transection of metatarsal bones. (c) Dissection of fat pad while retracting forefoot. (d) Creation of plantar flap. (e) Ligation of digital arteries (f) Skin closure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree