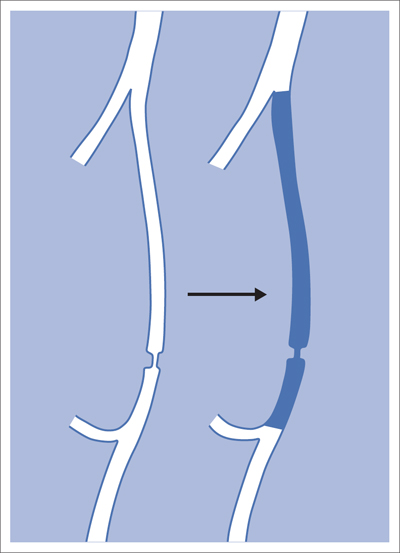
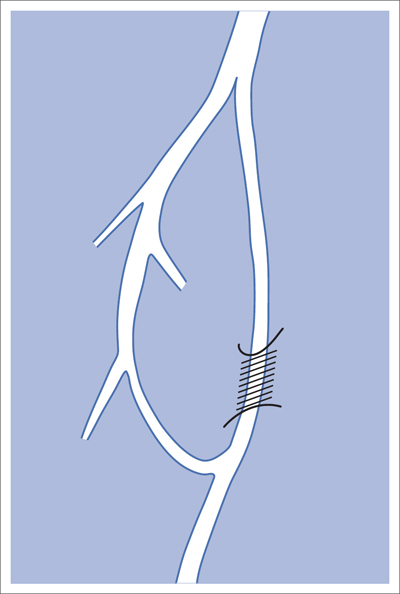
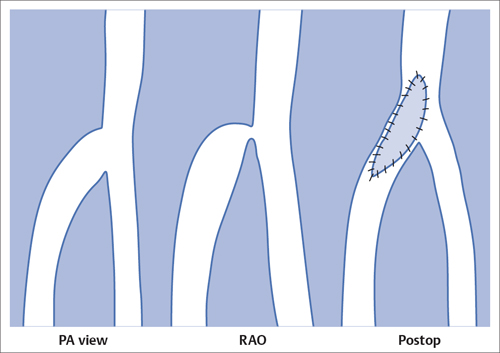
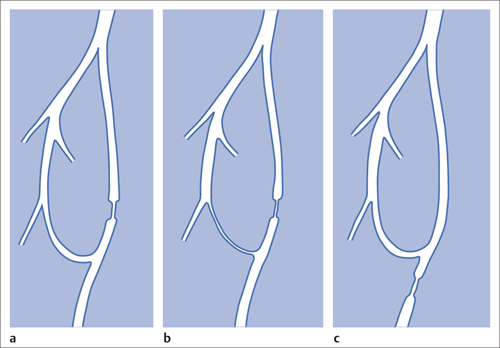
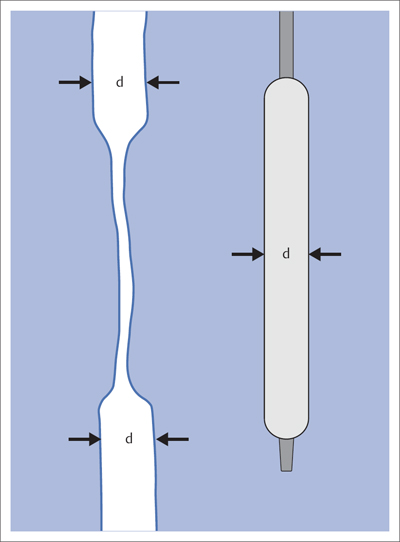
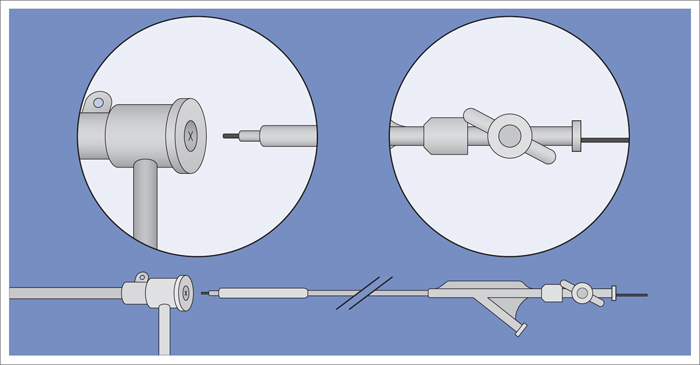
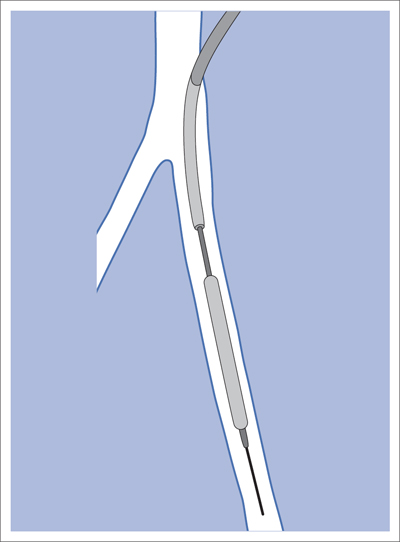
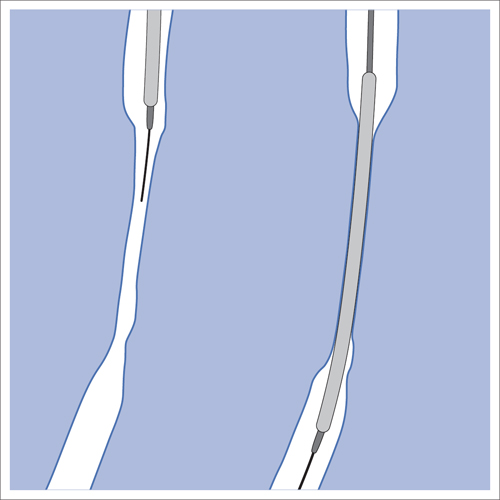
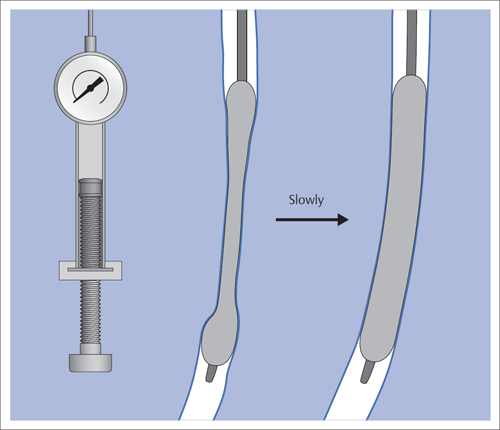
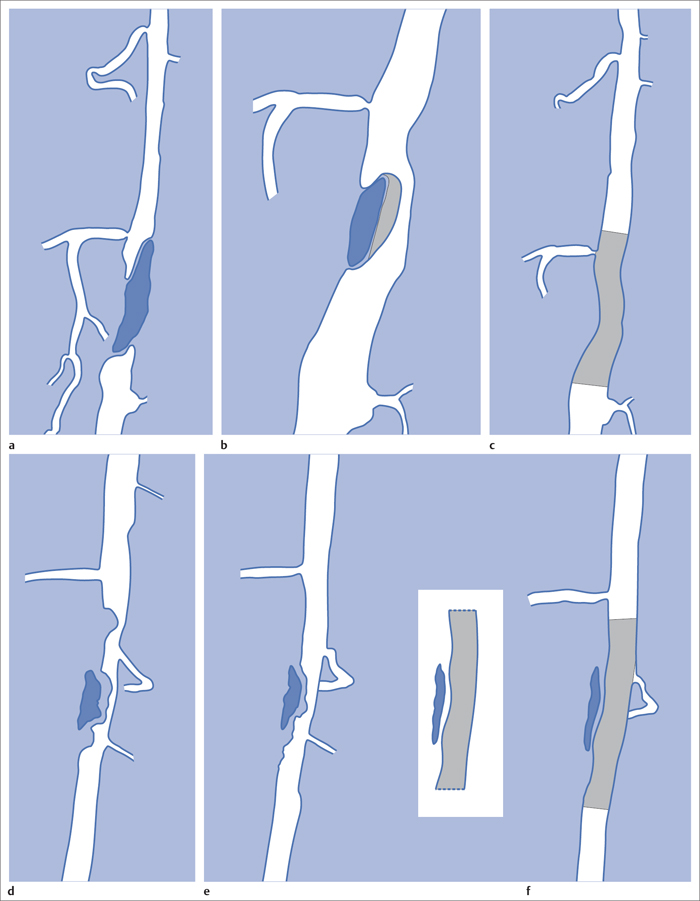
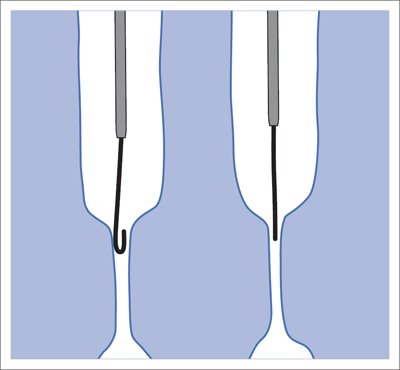
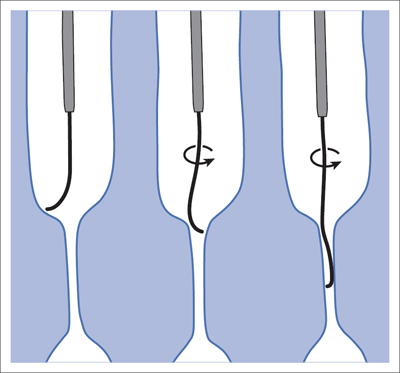
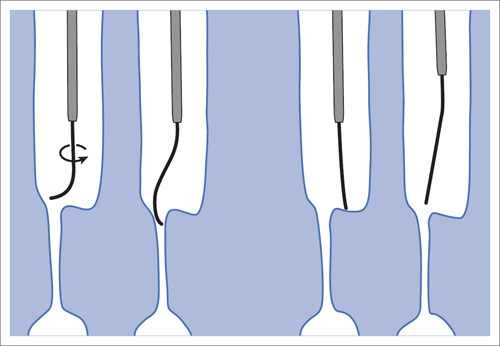
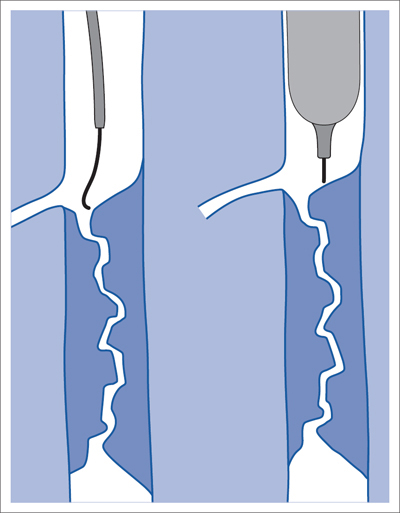
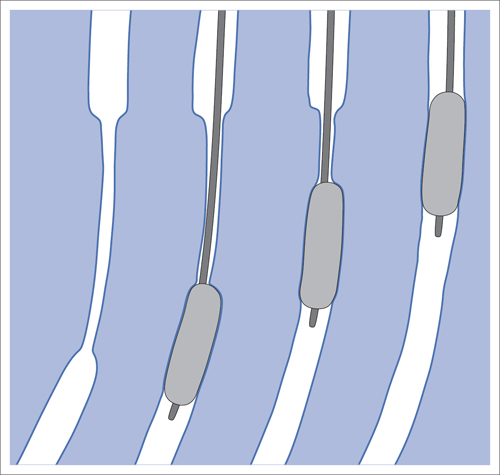
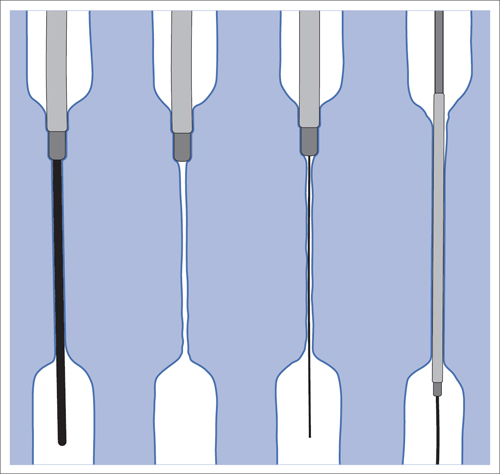
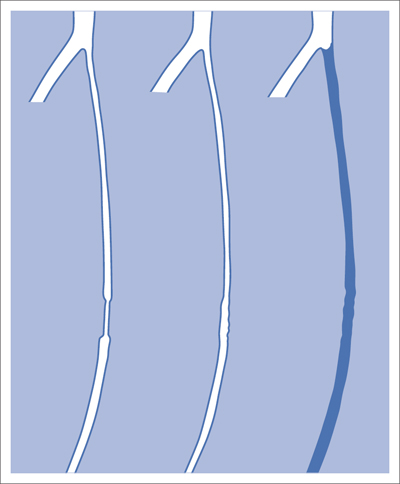
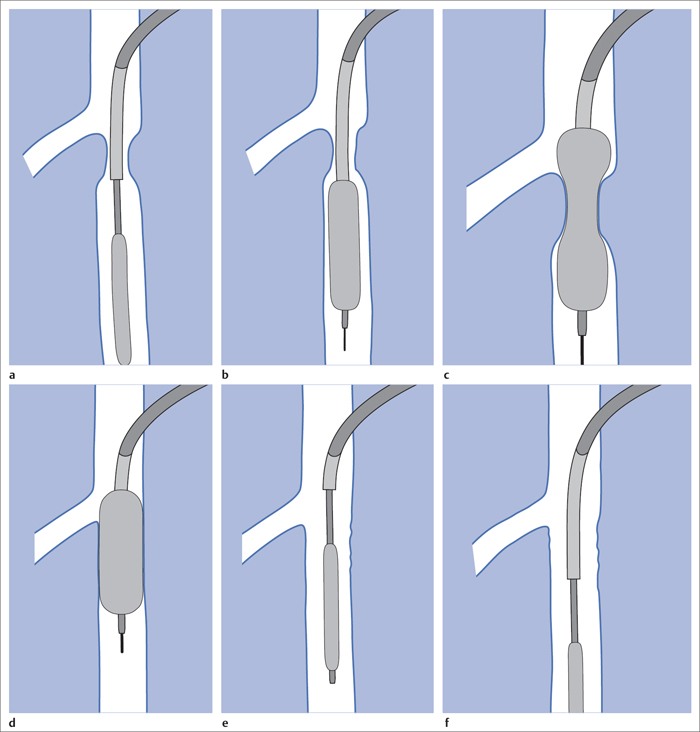
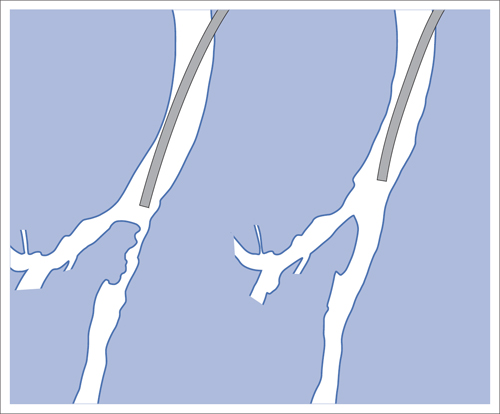
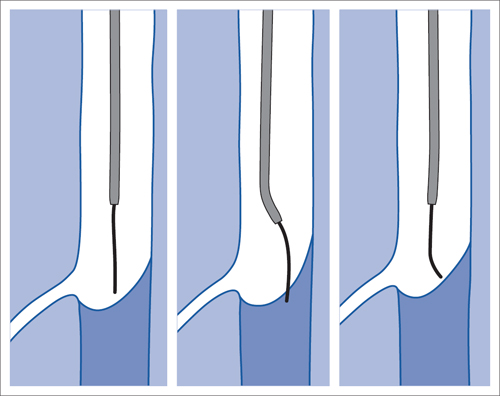
5 Lower Extremities Problems in the common femoral artery are treated primarily by surgery. The vessel courses superficially and is readily accessible. Surgery may be performed under local anesthesia if necessary. The often large eccentric plaques in the common femoral artery are poorly manageable by percutaneous transluminal angioplasty (PTA). Placement of a stent is contraindicated due to the motion in the groin and because a stent would prevent the use of the vessel for arterial access in any future endovascular procedures. The superficial femoral artery is an unusual vessel in many respects. • It usually has only a few insignificant branches or none at all over a long distance. This means that a short stenosis can lead to thrombotic occlusion of the entire vessel (Fig. 5.1). • Its distal segment is subject to mechanical stresses in the adductor canal. This may be why it is the peripheral artery most often affected by stenosis or occlusion (Fig. 5.2). • Parallel to the superficial femoral artery there is the large deep femoral artery. In many cases this artery has such strong connections to the superficial femoral and popliteal arteries that it can fully assume the function of an occluded superficial femoral artery. If this succeeds it is the most reliable long-term resolution of claudication. One of the most important diagnostic tasks is to avoid missing a correctable stenosis at the origin of the deep femoral artery (Fig. 5.3). • On the other hand in every intervention one must take care not to compromise the junction of the deep femoral artery collaterals with the superficial femoral artery. Correct evaluation of these collaterals can determine whether intervention in the superficial femoral artery is indicated. Yet there are certainly cases in which PTA in the superficial femoral artery helps to buy time until the deep femoral artery collaterals are fully developed (Fig. 5.4a, b). • Stenoses distal to the junction of the deep femoral artery collaterals are of special importance. In contrast to stenoses located farther proximally, they cannot be compensated by the collaterals (Fig. 5.4c). More than half of all cases of peripheral arterial occlusive disease involve the superficial femoral or popliteal artery. In spite of all the advances in interventional technique, the long-term results achieved with the venous bypass have been better than those of endovascular treatment. The results reported in the literature vary greatly. For example, a prospective study published in 2001 reported primary patency rates post-PTA of 87% after 1 year, 80% after 2 years, and 55% after 4 to 5 years (Clark et al 2001), whereas some authors observed primary patency rates of only 33 to 45% after 12 months (Dake 2011). Nevertheless, endovascular therapy remains the therapy of first choice for most cases. There are several reasons for this: • The bypass operation represents a significantly more invasive procedure with higher mortality and associated risks, for example, those associated with harvesting the venous graft. • Suitable veins are not always available and may also be required for coronary bypasses. • Endovascular treatments can be repeated and do not exclude a subsequent bypass. The most direct, quickest, and simplest approach to the leg arteries is the antegrade approach via the common femoral artery (see also Chapter 3, Antegrade Puncture of the Common Femoral Artery, p. 52). This applies to 80 to 90% of all cases. Even direct antegrade catheterization of the superficial femoral artery distal to the femoral bifurcation is an option in rare cases where the superficial femoral artery is wide enough. This may be the case in the presence of a high bifurcation of the common femoral artery or an abdomen that extends far caudally if crossover catheterization appears difficult or is unsuccessful. Obesity, a high bifurcation of the common femoral artery, or iliac arteries that also require treatment will necessitate crossover catheterization in 10 to 20% of cases (see Chapter 3, Crossover Catheterization, p. 60). The approach via the brachial artery (see Chapter 3, Access via the Brachial Artery, p. 65) or, very rarely, the popliteal artery is only occasionally required. Fig. 5.3 Stenosis at the origin of the deep femoral artery, visualized only on an ipsilateral oblique projection (center). Posteroanterior view (left). Treatment by patch reconstruction (right). Fig. 5.4 Significance of deep femoral artery collaterals in stenosis of the superficial femoral artery. a Superficial femoral artery stenosis compensated. b Not compensated. c Cannot be compensated. Interventional radiologists will hardly question the use of an ipsilateral antegrade approach to the arteries of the leg as standard practice. However, some cardiologists who also perform peripheral interventions declare crossover catheterization to be the normal approach. They also find reasons to make this sound plausible: 1. The compression of the groin can lead to reduced flow and when applied on the treated side could increase the risk of an acute thrombotic recurrence of the occlusion. This objection is not consistent with everyday experience, and it does not apply when the access site is treated with a vascular closure system. 2. Stenoses of the superficial femoral artery close to its origin are easier to treat via a crossover approach. That is correct. However, these usually do not pose any real problem even with an antegrade approach (see later discussion). Cardiologists’ preference for crossover catheterization is understandable when one considers that cardiological interventions use only retrograde catheterization. In this setting the crossover approach naturally provides an opportunity to address stenosis in a leg artery as well. There are several reasons why crossover catheterization should not be the technique normally used: • It takes longer and requires significantly more material. • The length of the approach through the iliac arteries renders manipulation difficult and limits one’s options (e.g., thrombus aspiration, see Chapter 3, Aspiration of Thrombus, p. 105). • A catheter bent at the bifurcation cannot be rotated around its longitudinal axis without resistance like an extended catheter can. In the bent segment of the catheter, the rotation causes internal deformation of the material. This consumes energy and is associated with resistance against the rotation (Schröder 1992). • Unjustified irritation of the iliac arteries is associated with the risk of cholesterol embolization. This risk may be slight, but in most cases it is avoidable. First select an appropriate sheath according to the following criteria: 1. Which instruments will be needed (guidewire, balloon catheter, stent)? When in doubt, check what is in stock. 2. Will it be possible to inject contrast through the sheath next to the balloon catheter? 3. How does one want to close the access site at the end of the intervention? The decision is easy when you have already opted for a vascular closure system. Then select the sheath that fits the vascular closure system, for example, 6 French for Angio-Seal (St. Jude Medical, St. Paul, MN, USA). In this case one can use a 5 French PTA catheter and still inject contrast through the sheath for road mapping and angiographic monitoring. If it becomes necessary to introduce a stent one will not have to replace the sheath to do so. Slender systems should be preferred where the access site is to be closed by external compression. Over the wire or monorail catheters that can be introduced over an 0.018 in. wire through a 4 French sheath are now available in all sizes commonly used in the superficial femoral artery. They are a good solution for all cases in which no difficulty in passing the wire or catheter is anticipated. In most catheters the shaft is so slender that it is also possible to inject contrast next to it. Where one has the choice of 9, 10, or 11 cm lengths, one should always choose 11 cm for an antegrade procedure. Then there will be less of a chance of the sheath slipping into the deep femoral artery from the superficial femoral artery when one changes the wire or catheter. • Perform angiography down to and including the arteries of the lower leg. • Administer a bolus of 5,000 IU of unfractionated heparin intra-arterially or intravenously. In small and very slender patients it may be advisable to reduce the dose to 3,500 or 4,000 IU. Do not administer heparin to patients with heparin-induced thrombocytopenia (HIT)! • Measure the vessel diameter and the length of the stenosis. • Select the appropriate balloon catheter: The diameter of the balloon should match the diameter of the vessel; the balloon should be 1 to 2 cm longer than the stenosis (Fig. 5.5). • Place a wire in the flushed catheter and lock it within the catheter with the stopcock (Fig. 5.6): The wire should project out of the tip of the catheter ~2 mm to facilitate introducing it into the sheath. • After introducing it into the sheath, advance the wire 1 to 2 cm further and again lock it (Fig. 5.7); advance the wire and catheter to within a short distance of the stenosis. • Perform road mapping via the sheath. • Introduce the wire and catheter into the stenosis under fluoroscopy (Fig. 5.8); remove the wire and flush the catheter and sheath with heparinized saline solution (flush intermittently during the entire treatment!). • Connect a manometer syringe (with contrast agent with 300 mg of iodine per mL, diluted 1:1) to the lumen of the balloon; slowly inflate the balloon under repeated fluoroscopic control (Fig. 5.9); slowly expanding the vessel reduces the risk of gross dissection. • Increase pressure to 6 to 8 bar over a period of 30 to 60 seconds. If one wants to tear tissue, it is best done with a sharp tug. But if one wants to expand it, one must increase the tension slowly and steadily. This gives the fibers in the tissue time to rearrange themselves in response to the tension. Dilating twice for 1.5 minutes is better than once for 3 minutes. This is because dilation creates a dead space proximal and distal to the balloon where the blood stagnates and can coagulate (Fig. 5.10). If the balloon tends to slip out of the stenosis, advance it far enough in that it tends to slip out distally. Then keep tension on the catheter. Some observations suggest that deformation and compression of the plaque material also contribute to the effect of PTA (Losordo et al 1992). For one thing, this would mean that moisture is being expressed. That is a process that takes time. Anyone with the patience to try to improve the results of PTA with longer and repeated dilation using the same balloon and the same pressure can observe repeatedly that this does indeed improve the results. Therefore, as a matter of course, it is best to dilate for longer than ~30 seconds. This spares all those patients requiring a second dilation unnecessary contrast administration. Moreover it spares them a second catheterization of the stenosis with the slight additional risk of dissection or thrombus mobilization (in the early days of PTA the watchword was “do not reenter!”). And cost-effectiveness is hardly the only reason why it is better to achieve a good result before having to switch to a larger balloon. Dilating for a little longer invests maybe an additional 3 minutes in the decisive phase of the treatment, which is hardly noticeable compared with the length of the procedure as a whole. Yet, to do so increases the probability of achieving good results and minimizes the risks. • Maintain pressure for 1.5 minutes; flush the catheter. Compare the balloon diameter to the vessel diameter (road mapping), then deflate the balloon. • When reinflating the balloon, observe whether it expands completely at low pressure (1 to 2 bar). • Perform a second dilation at 6 to 8 bar over a period of 1.5 minutes. Then the balloon catheter is withdrawn and results are evaluated by angiography performed via the catheter. Injecting contrast via the catheter will provide better image quality with the same amount of contrast agent. Good results (residual stenosis < 20%): Monitor lower leg arteries then finish intervention. Residual stenosis >20%: Repeat PTA at a higher pressure (such as 14 bar) using a balloon with compliance, or repeat the dilation with a larger balloon (possibly a shorter balloon adapted to the length of the residual stenosis). Longer duration of PTA (5 to 15 minutes) will often help in the case of an irregular and calcified residual stenosis. Another solution is to place a stent. Gross dissections that interfere with the flow of blood and residual stenoses > 30% that do not respond to treatment justify placement of a self-expanding stent. Wherever possible the stent should be no longer than the lesion itself. Caution: Only self-expanding stents should be used in the superficial femoral artery. Balloon-expandable stents can be permanently deformed by external pressure! Leg movement also causes changes in the length of the superficial femoral artery that only a self-expanding stent can adapt to. The suggestion of introducing the balloon catheter with a straight wire in the very first step of the procedure deviates from the customary routine. Most authors recommend first advancing a straight or curved 0.035 in. glide wire or 0.018 in. guidewire with a hydrophilic tip (also straight or slightly curved). The wire should be fed through a diagnostic catheter (straight or slightly curved) with a Tuohy-Borst adapter at its proximal end (see Fig. 2.45). The adapter seals the catheter lumen against the wire and at the same time allows contrast injections. These facilitate orientation by visualizing the current position of the wire and catheter. The balloon catheter is introduced only when the wire and diagnostic catheter have been successfully advanced through the stenosis. Fig. 5.10 Two examples of massively calcified eccentric residual stenoses that are only satisfactorily expanded by stent placement. a Subtotal occlusion, massive calcification. b Eccentric residual stenosis after percutaneous transluminal angioplasty (PTA). c Good expansion with nitinol stent. d Severely calcified eccentric stenosis. e PTA fails to bring decisive improvement. f Good result with nitinol stent. My experience has shown that it is just as easy to advance a balloon catheter through the lesion as a straight or curved diagnostic catheter. The straight wire is in most cases clearly superior to the curved wire. It can better negotiate narrow stenoses than a curved wire, the tip of which usually becomes stuck at the beginning of the stenosis and then causes the wire to form a loop (Figs. 5.11 and Fig. 5.12). For an eccentric stenosis one can give the wire a slight bend ~2 to 3 cm from its tip; this enables one to guide the otherwise straight tip of the wire into the opening of the stenosis using road mapping (Fig. 5.13). Occasionally one will encounter a very irregular stenosis through which the remaining lumen describes an irregular zigzag course. The lumen may contain dead ends that are only visualized on a different projection. It is practically impossible to formulate helpful general recommendations for catheterizing such stenoses. One can attempt to follow the lumen visualized by road mapping using a glide wire with a sharp bend immediately (2 mm) behind its tip (Fig. 5.14). If this is unsuccessful, you can best negotiate the stenosis by inflating the balloon to center the catheter within the lumen and then advancing the wire with the stiff end through the occlusion (Fig. 5.14). Fig. 5.14 Advancing a wire through a complicated stenosis is occasionally easier with the hard end of the wire centered in the lumen by the balloon catheter (right). Stenoses that are longer than the available balloon are dilated incrementally in overlapping segments from distal to proximal (Fig. 5.15). PTA with a balloon of sufficient length is quicker and better. Occasionally a stenosis will be so narrow and rigid that a 5 French catheter cannot be advanced through it (Fig. 5.16). In this case leave the catheter in place and introduce an 0.018 in. wire. Over this wire advance a 3 or 3.5 French catheter into the stenosis. This technique invariably works, and not only because of the smaller diameter of the balloon catheter. The 0.018 in. wire is also ~4 times as stiff as a normal 0.035 in. glide wire (see Chapter 2, Guidewires, p. 8). The risk of a superficial femoral artery measuring only 2 to 3 mm in diameter becoming occluded over its entire length following PTA is relatively great (Fig. 5.17). This may change stage IIb disease to stage III, an indication for a femoropopliteal bypass. The excessively narrow recipient vessel could cause occlusion of the bypass, in turn causing the patient to lose the lower leg, unquestionably a very poor outcome. Fig. 5.16 Passing a wire through a very hard, narrow stenosis. From left to right: 0.035 in. wire and 5 French catheter, 0.018 in. wire, 3.5 French catheter. Fig. 5.17 Dilating a superficial femoral artery that is very narrow over a long distance can lead to occlusion. Therefore, in the absence of pain at rest or peripheral emboli that would dictate active intervention, conservative management with gait training and supportive medical therapy is probably the better alternative. Convincing the patient of this will often require some effort. However, one will thus avoid a serious risk while giving collaterals from the deep femoral artery the chance to become strong enough that the symptoms finally disappear. The indication is entirely different where a vessel with a diameter of at least 4 mm is present. Here the risk of an acute occlusion is significantly less and early elimination of the stenosis can prevent the imminent occlusion of a long segment of the superficial femoral artery (Fig. 5.17). Thrombi can develop downstream of a stenosis or unstable plaque, become mobile, and occlude peripheral arteries as emboli. When this occurs, the patient presents with painful blue discoloration of sudden onset. This relatively rare clinical picture can be quickly eliminated by dilating the causative stenosis. In the case of an unstable plaque (note ultrasound findings), one may consider placing a covered stent. However, the search for the causative lesion can be difficult. When the toes of both feet are affected, one may expect to find the source of the embolism proximal to the aortic bifurcation. PTA of a stenosis at the origin of the superficial femoral artery is difficult to perform via an antegrade approach due to the proximity of the access site in the common femoral artery. For this reason most authors use crossover catheterization as a matter of course when treating the superficial femoral artery. The problem is that one must reliably avoid pulling the sheath out of the vessel and, more importantly, dilating the opening in the vessel wall as well. The solution is precision work guided by road mapping (Figs. 5.18 and 5.19). Perform road mapping via the sheath using an ipsilateral oblique projection to visualize the femoral artery bifurcation. • Inflate the balloon with low pressure and draw it up to the sheath. Fig. 5.18 Stenosis at the origin of the superficial femoral artery. a Road mapping. b Withdraw balloon back to sheath. c Pull balloon into stenosis. d Dilate. e Angiography. f Advance sheath. Fig. 5.19 Stenosis at the origin of the superficial femoral artery; 94-year-old woman with stage IV disease and erysipelas. Result of PTA (left). • Withdraw the balloon and sheath under fluoroscopy (road mapping) until the balloon lies within the stenosis (yet with the sheath still in the vessel). • Perform PTA (dilation). • Deflate the balloon and push it away from the entrance to the sheath; perform angiography via the sheath to evaluate results. • Advance sheath and catheter together until the sheath again lies securely within the superficial femoral artery. As to the question of how occluded segments of the superficial femoral artery should be catheterized, the literature provides a wide variety of opinions, some of them very imprecise. The most common recommendation is to use a wire with a hydrophilic coating, usually with a curved tip, guided by a straight or curved catheter. It may be possible to maneuver a slightly curved wire tip first through the stenoses, now no longer visible, that have caused the occlusion. This requires the use of a torqueable wire like a 0.035 in. glide wire or an 0.018 in. steel wire with hydrophilic coating on its tip. More probably, the tip of the wire will usually become stuck proximal to the stenosis and a U-shaped bend must then be forced through the stenosis, or the wire will deviate from the lumen and enter the wall. A curved catheter makes it easier to correct the direction of the wire yet it also makes it difficult to advance the catheter in a straight line. Most authors recommend initially advancing a straight or curved glide wire that is guided and steered by a catheter with a slight curve near its tip through an occluded segment (Figs. 5.20 and 5.21). Other authors reject the 0.035 in. glide wire in favor of an 0.018 in. wire with hydrophilic coating on its tip, to which many of them give a slight bend. The “snowplow” technique, advancing a van Andel catheter (Cook Medical, Bloomington, IN, USA) (straight, relatively stiff, tapered near the tip) from which projects the curvature of a Rosen wire (stiff steel wire, 1.5 mm radius of curvature), is somewhat similar to the technique the author suggests below. However, the disadvantage is that the curvature of a standard steel wire glides significantly less easily than the tip of a glide wire and certainly is more likely to deviate from the center of the vessel. These methods most often fail because the wire deviates into the vessel wall within the occluded segment. When advanced further, it usually spirals around the occluded lumen as the resistance increases (Fig. 5.22). Correcting the direction of advance with the curved catheter can be difficult. This is because the occlusion obscures vision and because only right and left deviations are detectable, not anterior or posterior deviations. The superficial femoral artery courses for the most part in a straight line. If it is no longer possible to bypass impediments in an occluded segment, then it is safest to negotiate the occlusion by remaining in the center of the vascular lumen and advancing in a straight line. This means using a straight wire and straight catheter. It is best not to bypass local impediments. A relatively stiff catheter is preferable. The soft tip of the wire is kept in the center. It will be less likely to deviate into the wall if it projects only a few millimeters from the catheter. If one uses the balloon catheter for this initial step instead of the curved catheter, then one will already have a straight and relatively stiff system of catheter and wire that will tend to remain along the axis of the vessel (Figs. 5.23 and 5.24).
Common Femoral Artery
Superficial Femoral Artery
Anatomy and Indication for Treatment
Long-Term Results
Approach
Performing the Procedure (Stenosis)
Why increase the pressure slowly?

Discussion of Catheterization Technique
Very Narrow and Hard Stenosis
Short Stenosis in a Superficial Femoral Artery with a Long, Narrow Segment
Blue Toe Syndrome
Stenoses at the Origin of the Superficial Femoral Artery
Occlusions of the Superficial Femoral Artery
Catheterization Recommendations in the Literature
Alternative Catheterization Technique
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Lower Extremities
Only gold members can continue reading. Log In or Register to continue