Low-Molecular-Weight Heparin Therapy during Acute Coronary Syndromes and Percutaneous Coronary Intervention
John J. Young
Dean J. Kereiakes
The past decade has seen major advances in adjunctive pharmacotherapy for acute coronary syndromes (ACS) and percutaneous coronary intervention (PCI). Pharmaco-therapeutic advances have resulted from a greater understanding of the pathophysiologic mechanisms underlying platelet activation and aggregation, thrombin generation, and thrombus formation. Thrombosis plays a central role in the pathogenesis of the acute ischemic syndromes and the complications of PCI. Rupture of atherosclerotic plaque initiates thrombosis and occurs spontaneously in ACS or is induced by percutaneous revascularization devices. Plaque rupture exposes thrombogenic subendothelial components that lead to platelet aggregation and initiation of the coagulation cascade. The propagation of thrombus may lead to partial or complete arterial occlusion and end-organ ischemia or infarction. Moreover, platelet-thrombus formation may be important in the progression of stable atherosclerotic plaque. In addition to cellular migration and proliferation, episodic plaque rupture, thrombus formation, and incorporation of thrombus into the plaque may contribute to the gradual, step-wise progression of arterial lumen compromise. Antithrombotic agents are, therefore, integral to management strategies for both ACS and PCI. Refinements in the dose regimens for use of unfractionated heparin (UFH), developments in the use of low-molecular-weight heparin (LMWH) and direct thrombin inhibitors (DTIs), as well as improvement in both oral and parenteral adjunctive antiplatelet therapies have occurred. This chapter reviews LMWH therapy for both ACS and during PCI.
UNFRACTIONATED HEPARIN
Unfractionated heparin (UFH) is a glycosaminoglycan, composed of a heterogeneous mixture of molecules of different molecular weights. The anticoagulant action of UFH
is derived from the binding of approximately one-third of UFH molecules to antithrombin III (AT-III), leading to a conformational change that markedly enhances the affinity of the UFH AT-III complex for activated thrombin (1). UFH avidly binds to endothelial cells, macrophages, and plasma proteins, which results in two distinct clearance phases from the body. An initial rapid equilibration phase is followed by a slower, saturable elimination phase (2). The binding capacity for UFH varies by individual, because heparin-binding proteins are largely acute-phase reactants. Thus, the half-life of UFH is both variable and dose dependent (1). Natural inhibitors of UFH (platelet factor 4 [PF4]) may be released from platelet-rich thrombus and may further alter the UFH dose-response relationship, especially when large amounts of thrombus are present (1).
is derived from the binding of approximately one-third of UFH molecules to antithrombin III (AT-III), leading to a conformational change that markedly enhances the affinity of the UFH AT-III complex for activated thrombin (1). UFH avidly binds to endothelial cells, macrophages, and plasma proteins, which results in two distinct clearance phases from the body. An initial rapid equilibration phase is followed by a slower, saturable elimination phase (2). The binding capacity for UFH varies by individual, because heparin-binding proteins are largely acute-phase reactants. Thus, the half-life of UFH is both variable and dose dependent (1). Natural inhibitors of UFH (platelet factor 4 [PF4]) may be released from platelet-rich thrombus and may further alter the UFH dose-response relationship, especially when large amounts of thrombus are present (1).
Controversy exists with regard to the appropriate periprocedural dosing and strategies to monitor procedural UFH therapy in clinical practice. The three main tests used to monitor UFH anticoagulation have been the whole blood clotting time, the activated clotting time (ACT), and the activated partial thromboplastin time (aPTT) (3, 4, 5). The term activated refers to the use of agents that activate factor XII during performance of the assay. The whole blood clotting time may be less reproducible and is more dependent on the degree of contact activation (3, 4, 5, 6). The aPTT traditionally has been used to monitor UFH therapy but has limited responsiveness to changes in UFH concentration, especially at higher levels of anticoagulation (7, 8, 9, 10). In addition, the use of a platelet-poor preparation during the measurement of the aPTT ignores the potential UFHneutralizing effects of PF4 (1). These concerns are less applicable to measurement of the ACT. The added appeal of a rapidly available “point-of-care” assay has contributed to making the ACT the standard for assessing UFH anticoagulation in catheterization laboratories (11,12).
Guidelines for UFH use during PCI have been the subject of discussion and controversy (13, 14, 15, 16). Initially, empirically derived intravenous (IV) bolus doses of 5,000 to 10,000 units of UFH prior to PCI were utilized widely. The variable adequacy of anticoagulation with this regimen led to the subsequent formation of weight-adjusted bolus dose regimens and to dose adjustment based on close monitoring of in-laboratory ACT values (11,17). Early recommendations for UFH dosing during PCI came from studies demonstrating that an ACT of more than 300 to 400 seconds is required to prevent fibrin deposition within the extracorporeal circuit in patients undergoing cardiopulmonary bypass (18). Retrospective analyses of clinical series demonstrated an inverse relationship between the intensity of UFH anticoagulation during PCI, as reflected by the ACT, and the occurrence of ischemic complications including abrupt vessel closure, emergency bypass surgery, and death (19, 20, 21, 22, 23, 24, 25, 26). However, the risk of serious bleeding complications also increased at progressively higher ACT levels (20,24,27,28). Although an optimal “target” or threshold for ACT could not be identified from these data, the consensus recommendation was made for achieving an ACT level ≥300 seconds during PCI.
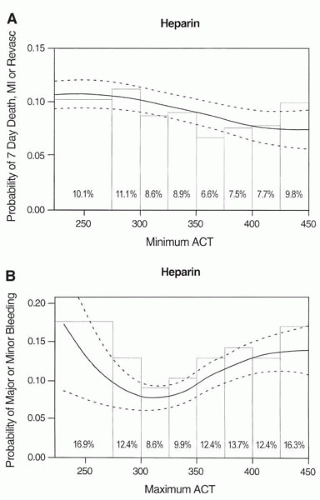
Recently, a meta-analysis that combined data from six randomized controlled trials was performed to determine the relationship of periprocedural ACT with clinical outcomes (29). This analysis included 6,146 patients derived from studies of novel adjunctive antithrombotic regimens, in which UFH constituted the control arm and ACT data were available on 5,216 patients. An ACT of 350 to 375 seconds during PCI was associated with the lowest composite event rate (death, myocardial infarction [MI], revascularization) to 7 days follow-up (6.6%) (29). The lowest incidence of major or minor bleeding events was observed for ACTs in the range of 300 to 325 seconds (8.6%), and progressively
increased at 350 to 375 seconds (12.4%) (Fig. 47.1A,B). This analysis concluded that, contrary to prior reports, the optimal suppression of ischemic events with UFH therapy in patients undergoing PCI requires ACT levels substantially higher than previously appreciated. These data suggest that the optimal level of periprocedural ACT is in the range of 350 to 375 seconds for preventing ischemic complications while minimizing bleeding complications during PCI.
increased at 350 to 375 seconds (12.4%) (Fig. 47.1A,B). This analysis concluded that, contrary to prior reports, the optimal suppression of ischemic events with UFH therapy in patients undergoing PCI requires ACT levels substantially higher than previously appreciated. These data suggest that the optimal level of periprocedural ACT is in the range of 350 to 375 seconds for preventing ischemic complications while minimizing bleeding complications during PCI.
The dose of UFH should be reduced using a weight-adjusted algorithm if concomitant platelet glycoprotein (GP) IIb/IIIa receptor inhibitor therapy is administered. Through an interaction at the level of the GP IIb/IIIa receptor, GP IIb/IIIa blocking agents further prolong the ACT in the presence of UFH (30,31). In the Evaluation of c7E3 for the Prevention of Ischemic Complications (EPIC) trial, markedly elevated ACT levels were observed following the administration of non-weight-adjusted UFH in combination with abciximab and were associated with frequent major bleeding complications and transfusion requirements (32). Subsequent utilization of a reduced-dose weight-adjusted UFH regimen in conjunction with early vascular access sheath removal resulted in a marked reduction in both bleeding and transfusion events for abciximab-treated patients (33). The safety and efficacy of this reduced-dose UFH regimen (70 U/kg bolus) in combination with abciximab was subsequently confirmed in a large, multicenter randomized trial (Evaluation of PTCA to Improve Long-Term Outcome by c7E3 GP IIb/IIIa Receptor Blockade [EPILOG]) (34). Mean and median maximum in-laboratory ACT values observed with this regimen were 270 and 299 seconds, respectively. Although subsequent clinical trials and empirical clinical experience have suggested enhanced safety (fewer bleeding complications) with maintenance of efficacy at even lower doses of weight-adjusted UFH without concomitant GP IIb/IIIa blockade (65 U/kg or 60 U/kg UFH) (35,36) or with concomitant GP IIb/IIIa blockade (50 U/kg or less [≤1,000 units] UFH) (37,38) to target ACT values of 200 seconds during PCI, these regimens have not been compared in large-scale randomized trials. Most recently, a pooled analysis involving data from four randomized controlled trials of adjunctive pharmacotherapy for PCI involving a high prevalence (89%) of platelet GP IIb/IIIa receptor blockade and bivalirudin use observed no relationship between ACT prolongation and clinical ischemic events. Interestingly, although no relationship between ACT and major bleeding events was observed, the composite occurrence of major and minor bleeding events was increased in the highest (3rd and 4th) quartiles of periprocedural ACT (39).
The Organization to Assess Strategies for Ischemic Syndromes (OASIS) investigators recently examined the relationship between aPTT, recurrent cardiovascular events, and bleeding among 5,058 patients receiving UFH during ACS (40). There was an increase in relative risk of cardiovascular events (1.54, 95% CI 1.10 to 2.15; p = 0.01) among patients with subtherapeutic (<60 seconds) aPTT values, while higher aPTT values were associated with increased bleeding; for every 10-second increase in aPTT, the probability of major bleeding was increased 7% (95% CI 3% to 11%; p = 0.0004) (40). These results justify the practice of regularly monitoring UFH and substantiate the difficulty in maintaining therapeutic levels with this anticoagulant. The administration of an LMWH may provide a more reliable and safe means of antithrombin therapy during ACS and PCI.
LOW-MOLECULAR-WEIGHT HEPARIN
LMWHs are produced by enzymatic or chemical depolymerization of UFH and have saccharide chains with molecular weights of 4,000 to 6,500 Daltons (average 5,000 D or 15 saccharide units) (41, 42, 43). Due to their relatively small molecular size, LMWHs have a greater capacity for inactivating factor Xa, relative to thrombin (factor IIa). Thus, compared with UFH, which has a ratio for factor Xa to IIa inactivation of 1:1, LMWHs exhibit antifactor Xa to IIa ratios of between 2 and 4:1 (43). Because early (“upstream”) events are greatly magnified during the coagulation cascade, factor Xa likely contributes more to the procoagulant activity of thrombus in situ than does thrombin (IIa). Furthermore, inhibition of factor Xa may be of greater importance in ACS and during PCI.
Unlike UFH, LMWHs have little nonspecific plasma protein and cellular binding, which contributes to a more predictable dose response and a longer pharmacologic half-life. Furthermore, the bioavailability of LMWH following subcutaneous (SQ) injection approximates 90% (versus 30% for UFH) (43). This combination of more predictable anticoagulant response, high bioavailability, and long half-life suggests that a reliable anticoagulant response can be achieved following twice-daily SQ injections using a weight-adjusted dose regimen in the absence of routine laboratory monitoring. In addition, animal models of thrombosis have shown LMWH to be associated with less bleeding (enhanced safety), which may in part be explained by a lower affinity for platelets and less consequent inhibition of platelet function (43). Heparin-induced thrombocytopenia also occurs less commonly with LMWH than UFH. The incidence of antiplatelet factor 4/heparin antibody induction in patients undergoing PCI with UFH is higher than previously recognized (44). These potential advantages (Table 47.1) of LMWH over UFH in terms of clinical efficacy, safety, and ease of use in the setting of ACS and PCI have prompted evaluation in numerous studies.
Low-Molecular-Weight Heparin in ST-Elevation Myocardial Infarction
Several studies have evaluated the efficacy of LMWH in the setting of ST-elevation myocardial infarction (STEMI). The
effectiveness of pharmacological reperfusion for STEMI depends on the balance between fibrinolytic and prothrombotic activity. Fibrinolysis enhances the prothrombotic state associated with STEMI by promoting the generation and release of thrombin following successful clot lysis, with consequent activation of the coagulation cascade that leads to further platelet activation (45,46). Therefore, sound theoretical rationale supports the use of concurrent antithrombotic therapy to enhance the process of fibrinolysis.
effectiveness of pharmacological reperfusion for STEMI depends on the balance between fibrinolytic and prothrombotic activity. Fibrinolysis enhances the prothrombotic state associated with STEMI by promoting the generation and release of thrombin following successful clot lysis, with consequent activation of the coagulation cascade that leads to further platelet activation (45,46). Therefore, sound theoretical rationale supports the use of concurrent antithrombotic therapy to enhance the process of fibrinolysis.
TABLE 47.1. ADVANTAGES OF LOW-MOLECULAR-WEIGHT HEPARIN OVER CONVENTIONAL UNFRACTIONATED HEPARIN | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The LMWHs dalteparin, enoxaparin, and, most recently, reviparin have been evaluated in combination with fibrinolytic therapy for AMI. Infarct-related arterial patency following fibrinolysis has been evaluated in three trials with enoxaparin: the Acute MI-Streptokinase (AMI-SK) (47), the second trial of Heparin and Aspirin Reperfusion Therapy (HART II) (48), and the Enoxaparin Antithrombin Therapy for ST-Elevation Thrombolysis in MI (ENTIRE-TIMI 23) (49); and two trials with dalteparin: the second trial of Biochemical Markers in ACSs (BIOMACS II) (50) and the ASSENT Plus (51). The use of adjunctive LMWH resulted in improved late coronary artery patency rates (3% to 16% absolute improvement in TIMI 2 flow and TIMI 3 flow) (47, 48, 49,51) and a tendency toward higher TIMI 3 flow rates (1% to 17% absolute improvement) (47, 48, 49, 50, 51) compared with UFH. Improvement in angiographic endpoints was associated with a reduction (odds ratio [OR] 0.74; 95% confidence intervals [CI] 0.51 to 1.09) in the composite occurrence of death or recurrent MI in the pooled analysis of these three trials. Adjunctive LMWH may also be associated with improved tissue-level perfusion following fibrinolysis, as assessed by the magnitude of ST-segment resolution and other noninvasive composite measurements of reperfusion seen with enoxaparin in the AMI-SK (47) and Accelerated Streptokinase and Enoxaparin (ASENOX) (52) trials, respectively. The rates of other significant clinical events, such as late infarct-related arterial reocclusion and recurrent ischemia, were reduced with LMWH compared with UFH, with overall similar bleeding rates (47, 48, 49, 50, 51, 52, 53).
The Assessment of the Safety of a New Thrombolytic 3 (ASSENT-3) study evaluated the efficacy and safety of full-dose tenecteplase plus enoxaparin or half-dose tenecteplase plus abciximab versus full-dose tenecteplase plus UFH in 6,095 patients (54). Significantly lower event rates were observed for the efficacy (30-day mortality, in-hospital reinfarction, or refractory ischemia) plus safety (efficacy plus intracranial hemorrhage or in-hospital major bleeding) composite endpoints in the enoxaparin and abciximab groups compared to the UFH group, primarily due to a reduction in ischemic complications. Thirty-day mortality rates were not different among groups (5.4% enoxaparin, 6.6% abciximab, 6.0% UFH; p = 0.25) and follow-up to 1 year demonstrated similar mortality (54,55).
More recent data from the ASSENT-3 PLUS study underscores the need for continued evaluation of the safety of LMWH as an adjunct to fibrinolysis (56). Among 1,639 patients with STEMI receiving tenecteplase and either enoxaparin or UFH in a prehospital setting, higher rates of both major bleeding (4.0% versus 2.8%) and intracranial hemorrhage (2.2% versus 1.0%; p = 0.05) were seen in the enoxaparin group compared with the UFH group (56). A significant interaction was noted between patient age and risk of bleeding, because the majority of cases of intracerebral hemorrhage were observed in patients older than 75 years. The safety concern of enoxaparin among elderly patients will be addressed in the ongoing Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment (ExTRACT)-TIMI 25 trial (57), which is a double-blind, parallel group trial randomizing ˜21,000 fibrinolysiseligible STEMI patients in a 1:1 fashion to receive either adjunctive UFH or enoxaparin, with dose reductions in patients over 75 years (57).
Recently, the Safety and Efficacy of Subcutaneous Enoxaparin versus Intravenous UFH and Tirofiban versus Placebo in the Treatment of Acute ST-Segment Elevation Myocardial Infarction Patients Ineligible for Reperfusion (TETAMI) trial demonstrated no benefit of enoxaparin over UFH in patients with STEMI who presented later than 12 hours from symptom onset (58). The primary endpoint (30-day composite occurrence of death, reinfarction, or recurrent angina) was not significantly different between groups (15.7% enoxaparin versus 17.3% UFH; 16.6% tirofiban versus 16.4% placebo) (58). Enoxaparin appeared to have a similar safety and efficacy profile compared to
UFH, but did not enhance late reperfusion. Finally, the Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation (CREATE) trial evaluated the effects of reviparin when initiated early and given for 7 days in 15,570 patients with STEMI (59). The primary composite endpoint (death, myocardial reinfarction, or stroke at 7 and 30 days) was significantly reduced (9.6% reviparin versus 11.0% placebo; p = 0.005), with a reduction in 30-day mortality (9.8% reviparin versus 11.3% placebo; p = 0.005) and no significant differences in stroke (1.0% reviparin versus 0.8% placebo; p = 0.19) (59). An increase in life-threatening bleeding at 7 days was observed with reviparin versus placebo, but the absolute excess was small in comparison to the reduction in the primary endpoint. This study concluded that reviparin administered early after STEMI symptom onset and maintained for 7 days reduced mortality and reinfarction without an appreciable increase in stroke, but with the cost of a small absolute excess in life-threatening bleeding events.
UFH, but did not enhance late reperfusion. Finally, the Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation (CREATE) trial evaluated the effects of reviparin when initiated early and given for 7 days in 15,570 patients with STEMI (59). The primary composite endpoint (death, myocardial reinfarction, or stroke at 7 and 30 days) was significantly reduced (9.6% reviparin versus 11.0% placebo; p = 0.005), with a reduction in 30-day mortality (9.8% reviparin versus 11.3% placebo; p = 0.005) and no significant differences in stroke (1.0% reviparin versus 0.8% placebo; p = 0.19) (59). An increase in life-threatening bleeding at 7 days was observed with reviparin versus placebo, but the absolute excess was small in comparison to the reduction in the primary endpoint. This study concluded that reviparin administered early after STEMI symptom onset and maintained for 7 days reduced mortality and reinfarction without an appreciable increase in stroke, but with the cost of a small absolute excess in life-threatening bleeding events.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree