A. Patients whose ventricular function is deemed unrecoverable or unlikely to recover without long-term device support
B. Patients who are deemed too ill to maintain normal hemodynamics and vital organ function with temporary MCS or who cannot be weaned from temporary MCS or inotropic support
C. Patients with the capacity for meaningful recovery of end-organ function and quality of life
D. Patients without irreversible end-organ damage
9.4 Rationale for Temporary VAD Support
In low INTERMACS profile patients in whom cardiac recovery is a possibility (e.g., patients with myocardial stunning following acute ischemia and reperfusion and those with fulminant myocarditis), it would probably be more appropriate to consider using temporary VAD support. A recent or evolving stroke is considered at least a temporary contraindication for implantable VAD. In emergency cases with uncertain neurology, a short-term VAD may be considered to allow for proper assessment of the neurologic status of the patient. In countries where temporary VAD support is an eligible criterion for the urgent heart transplant waiting list, even chronic heart failure patients with acute decompensation may be better off being supported with a temporary VAD, thus avoiding the long wait for a transplant once an implantable VAD is in place and its associated adverse events. If the waiting time for an urgent heart transplant is short in terms of days or weeks, temporary VAD support under these circumstances may actually prove to be more cost-effective as well (◘ Table 9.2).
Table 9.2
Indications for temporary VAD support in low INTERMACS profiles patients
Opportunity of myocardial recovery |
Myocardial stunning following acute ischemia and reperfusion |
Postcardiotomy |
Fulminant myocarditis |
Primary graft dysfunction post-heart transplant |
Ominous features while on ECMO support |
Severe pulmonary edema resulting in upper body hypoxia |
Nonejecting ventricles with stasis |
Unsuitable for primary durable VAD support |
Recent or evolving stroke |
Uncertain neurology following cardiac arrest |
Severe end-organ dysfunction with uncertain capacity for recovery |
Inadequate psychosocial support for home discharge |
9.5 Types of Temporary VAD Systems
Temporary VADs are being used more frequently in order to normalize cardiac output in patients struggling with severe heart failure and, often times, patients in cardiogenic shock. There is a shift in pharmacologic therapy to a more hybrid approach incorporating MCSD. Bridge to decision is an option that utilizes temporary methods not only to stabilize the patient but to bide time until more definitive therapy has been decided. Some of the more common options include both percutaneous and surgical approaches(◘ Table 9.3).
Table 9.3
Examples of temporary ventricular assist devices
Percutaneous devices |
TandemHeart |
Impella |
HeartMate PHP |
Surgical options |
Berlin EXCOR |
CentriMag |
TandemHeart system (CardiacAssist, Inc.) is an extracorporeal continuous-flow centrifugal pump which acts as a left atrial-to-femoral artery bypass (◘ Fig. 9.1). It consists of transseptal and arterial cannulae and a centrifugal blood pump which delivers flow rates up to 4 L/min at a maximum speed of 7500 rpm. Clinical trials [4–11] in the United States and Europe have provided evidence supporting an increase in cardiac index and mean arterial pressure and a subsequent decrease in pulmonary capillary wedge pressure.
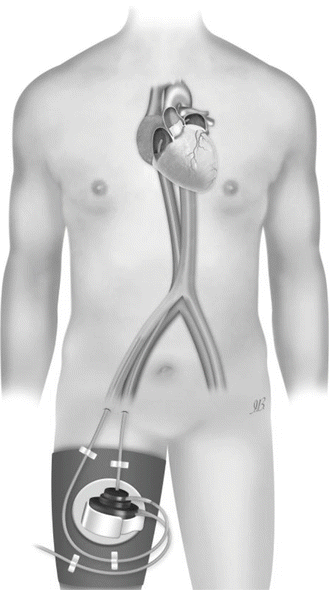

Fig. 9.1
TandemHeart system (CardiacAssist, Inc.) (Illustration by Ilaria Bondi’s Peppermint Advertising)
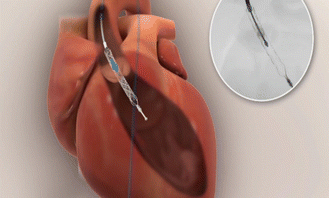
Impella (Abiomed) is another extracorporeal device used for partial circulatory support and for determining intravascular pressure. Available in 2.5, 3.5, and 5.0 L options, the Impella is a short-term axial flow device that draws blood from the left ventricle and empties it into the aortic root (◘ Fig. 9.2). Benefits include reducing end-diastolic volume and pressure, mechanical work, myocardial wall tension, oxygen demand, and overall cardiac output.

Fig. 9.2
Impella (Abiomed)
HeartMate PHP (percutaneous heart pump) (Thoratec Corporation) is a low profile, rapid insertion, catheter-based heart pump designed to provide 4–5 L/min of high forward flow to unload the left ventricle and fulfill end-organ perfusion. It is inserted through the femoral artery using a 14 French sheath and, once through the aortic valve, expands to 24 French for maximal flow (◘ Fig. 9.3). This device is currently under investigation in postcardiotomy patients weaning from cardiopulmonary bypass.