Although implantable cardioverter defibrillator (ICD) leads are prone to failure and Food and Drug Administration recall, comprehensive longitudinal studies investigating contemporary ICD lead survival rate in the United States are lacking. All patients receiving Medtronic, Boston Scientific, or St. Jude Medical transvenous ICD leads at the hospitals of the University of Pittsburgh Medical Center from 2000 to 2012 were included. Leads were classified as (1) functional lead, patient deceased; (2) functional lead, replaced; (3) failed lead, replaced; or (4) functional lead, active. Kaplan-Meier survival curves were constructed for all lead models separately and in aggregate. We followed 5,288 patients (1,020 Quattro, 623 Fidelis, 627 Riata, 828 Durata, and 2,190 Reliance) over 3.7 ± 3.3 years. Functional leads that were replaced included 30 Quattro (3%), 99 Fidelis (16%), 24 Riata (4%), 24 Durata (3%), and 62 Reliance (3%). Leads replaced because of failure included 11 Quattro (1%), 47 Fidelis (8%), 38 Riata (6%), 18 Durata (2%), and 26 Reliance (1%; p <0.001 for Food and Drug Administration recalled vs nonrecalled leads). Overall survival rate of all leads was 89.3% at 5 years. Survival curves of Riata and Fidelis diverged from nonrecalled leads at approximately 2 years. In conclusion, the overall survival rate of ICD leads is nearly 90% at 5 years. Survival curves of recalled leads diverge from nonrecalled leads after 2 years of implantation. These data have important implications on postmarket release monitoring of ICD leads and physicians’ choice of leads.
In the past 2 decades, indications for implantable cardioverter defibrillator (ICD) therapy have expanded significantly, particularly for primary prevention of sudden cardiac death. As patients who receive ICDs continue to live longer, dwell times of ICD systems are also becoming progressively longer. It is well accepted that the defibrillator lead represents a weak component of the ICD system because of its inherently complex design and continuous exposure to mechanical stress in situ. A few European studies have been published, demonstrating an increased incidence of lead failure at >5 years after lead implantation. Two ICD leads—the Sprint Fidelis (Medtronic Inc., Minneapolis, Minnesota) and the Riata (St. Jude Medical, Sylmar, California)—have been recalled by the Food and Drug Administration (FDA) in 2007 and 2011, respectively, after observed high rates of premature lead failure. Despite these FDA recalls, comprehensive studies investigating the overall ICD lead survival rate in the United States (US) are scarce. We therefore investigated the overall ICD lead survival rate of lead models from 3 major manufacturers implanted at the hospitals of the University of Pittsburgh Medical Center from 2000 to 2012.
Methods
All patients receiving a Medtronic, Boston Scientific, or St. Jude Medical transvenous ICD lead at the University of Pittsburgh Medical Center from November 2000 to March 2012 were included. Patients were followed in the outpatient device clinic with no <1 clinic visit and 3 remote checks/year or 2 clinic visits/year in patients with no remote home monitoring. Leads were classified as (1) functional lead, patient deceased (from any cause); (2) functional lead replaced for any reason other than lead failure (e.g., infection, heart transplantation, prophylactically after recall); (3) electrically failed lead, replaced; or (4) functional lead, active. Prophylactic replacement of recalled leads was performed on patient request and was offered primarily to high-risk patients (pacemaker-dependent or secondary prevention). Lead failure was defined as electrical malfunction resulting in lead extraction or replacement with a new ICD lead. Although no standard definition of ICD lead failure exists, we used criteria similar to those of previous reports. Specifically, electrical malfunction was defined as abnormal pace-sense or high-voltage impedance values, decrease in R-wave amplitude, increase in pacing threshold necessitating lead replacement; or the presence of electrical noise leading to inappropriate ICD therapy. Leads demonstrating mechanical failure (e.g., cable externalization in Riata leads) with normal electrical function were not classified as lead failures in the present study. Kaplan-Meier survival curves were constructed for all leads in aggregate and for each lead model separately.
All continuous variables are presented as mean ± SD and were compared using the Student t test. All categorical variables are presented as absolute numbers and percentages and were compared using the chi-square test. Lead survival rate was evaluated using the Kaplan-Meier method and compared between lead models using the log-rank test. A 2-sided p-value of 0.05 was considered statistically significant. All statistical analyses were performed on SPSS, version 19.0.0 (IBM Inc., Armonk, New York).
Results
A total of 5,288 patients (1,020 Sprint Quattro [models 6947 and 6935] [Medtronic Inc., Minneapolis, Minnesota], 623 Sprint Fidelis [model 6949], 627 Riata [models 1580 to 1582, 1590, 7000, 7001, 7011, and 7041], 828 Durata [models 7120 to 7122] [Saint Jude Medical, Sylmar, California], and 2,190 Reliance [models 0185 and 0158] [Boston Scientific, Natick, Massachusetts]) were included and followed for a mean of 3.7 ± 3.3 years. Baseline characteristics of all patients are detailed in Table 1 . Functional leads that were replaced included 30 Quattro (3%), 99 Fidelis (16%), 24 Riata (4%), 24 Durata (3%), and 62 Reliance (3%). Leads replaced for electrical failure included 11 Quattro (1%), 47 Fidelis (8%), 38 Riata (6%), 18 Durata (2%), and 26 Reliance (1%; p <0.001 for comparison of Fidelis and Riata with other lead models) as listed in Table 2 . Mode of lead failure is included in Table 2 .
| Characteristic | Quattro | Fidelis | Riata | Durata | Reliance |
|---|---|---|---|---|---|
| Number of leads | 1,020 | 623 | 627 | 828 | 2,190 |
| Age (yrs) | 64 ± 14 | 66 ± 13 | 66 ± 14 | 68 ± 15 | 72 ± 12 |
| Men (%) | 78 | 75 | 76 | 75 | 78 |
| CRT (%) | 38 | 41 | 36 | 44 | 38 |
| Follow-up (yrs) | 3.5 ± 2.7 | 2.7 ± 1.3 | 3.2 ± 2.1 | 2.3 ± 1.2 | 4.6 ± 2.7 |
| Lead Outcome | Quattro | Fidelis | Riata | Durata | Reliance |
|---|---|---|---|---|---|
| Functional lead, patient deceased, n (%) | 264 (26) | 77 (12) | 198 (32) | 46 (6) | 847 (39) |
| Functional leads replaced, n (%) | 30 (3) | 99 (16) | 24 (4) | 24 (3) | 62 (3) |
| Failed leads replaced, n (%) | 11 (1) | 47 (8) | 38 (6) | 18 (2) | 26 (1) |
| Mode of electrical failure | |||||
| Sensing/noise | 3 | 28 | 15 | 2 | 13 |
| Impedance change | 7 | 5 | 2 | 0 | 0 |
| High threshold | 0 | 4 | 7 | 2 | 6 |
| Perforation/dislodgement | 0 | 1 | 4 | 13 | 4 |
| High-voltage failure | 0 | 0 | 4 | 1 | 3 |
| Unknown mechanism | 1 | 9 | 6 | 0 | 0 |
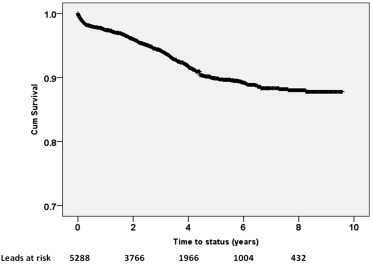
Overall survival rate of all leads was 89.3% at 5 years ( Figure 1 ). Individual failure-free survival curves were also generated for each ICD lead model, separately ( Figure 2 ). Survival curves for the 2 ICD leads under FDA recall (Riata and Sprint Fidelis) diverged from the other curves after nearly 2 years on the overall survival and failure-free survival Kaplan-Meier plots. Furthermore, lead models with the longest follow-up time, that is, Sprint Quattro and Endotak Reliance, exhibited 8-year survival rates of 98.3% and 98.5%, respectively.