The value of late gadolinium-enhanced (LGE) magnetic resonance imaging (MRI) for the prediction of functional recovery after surgical revascularization has been previously established. However, the impact of LGE-MRI on the long-term prognosis after coronary artery bypass grafting (CABG) remains incompletely understood. Therefore, we aimed to evaluate the long-term prognostic value of LGE-MRI, based on the presence or absence of left ventricular (LV) dysfunction, in patients with coronary artery disease undergoing CABG. One hundred forty-six consecutive patients underwent cine- and LGE-MRI before CABG. Adverse cardiac events included cardiac death, nonfatal myocardial infarction, heart failure, and unstable angina. A 3-year landmark analysis of the primary end point was also performed for patients surviving beyond 3 years after CABG. During a median follow-up of 9.4 years, 44 patients (30%) experienced adverse cardiac events. Although a LV ejection fraction <50% was associated only with adverse cardiac events at 3 years after CABG, LGE was associated with a worse outcome both at and beyond 3 years after CABG. In the overall study population, LGE presence (adjusted hazard ratio [HR] 2.58; p = 0.027), score (adjusted HR 1.06; p <0.001), and extent (adjusted HR 1.08; p <0.001) were independent predictors of adverse cardiac events. Moreover, in both the LV ejection fraction <50% and ≥50% groups, the LGE extent was an independent predictor of adverse cardiac events. In conclusion, our qualitative and quantitative analyses of LGE-MRI provide long-term prognostic information after surgical revascularization. The LGE extent was a strong predictor of adverse cardiac events, independent of the LV function.
Late gadolinium-enhanced (LGE) magnetic resonance imaging (MRI) can accurately delineate irreversible myocardial injury associated with adverse cardiac events. Furthermore, LGE-MRI enables evaluation of the transmural scar extent and allows prediction of functional recovery after coronary artery bypass grafting (CABG) surgery. However, no previous study has evaluated the prognostic value of LGE-MRI after revascularization. Gerber et al. reported that the detection of dysfunctional viable myocardium by LGE-MRI was an independent predictor of mortality in medically treated patients but not in revascularized patients. However, their study included only patients with severe left ventricular (LV) dysfunction, and survival was compared only according to the presence or absence of myocardial viability based on the transmural extent of LGE during the midterm follow-up. Herein, our primary aim was to evaluate the long-term prognostic value of LGE-MRI compared to that of the left ventricular ejection fraction (LVEF) in patients undergoing CABG. In addition, we evaluated the prognostic values of the LGE presence, score, and extent according to the presence or absence of LV dysfunction.
Methods
The medical records of 220 consecutive adult patients who underwent CABG from June 2003 to July 2005 at our institution were retrospectively reviewed ( Figure 1 ). One hundred sixty-five patients underwent cardiac MRI to evaluate the myocardial viability within 1 month before CABG. Of these, 19 patients were excluded because of hypertrophic cardiomyopathy, significant valve disease (grade ≥2 stenosis or insufficiency), or uninterpretable MRI findings. The remaining 146 patients formed the study cohort. The institutional review board approved this study (B-1407-258-104) and waived the need for written informed consent.
A 1.5T MRI system (Intera CV release 10; Philips Healthcare, Bothell, Washington) with 5-channel cardiac coils was used. All images were acquired with electrocardiogram gating and breath-holding. Steady state free-precession cine-MRI included vertical long-axis images, 4-chamber view images, and a set of short-axis images covering the entire left ventricle. The sequence parameters were as follows: field of view 350 to 400 mm; repetition time/echo time 3.0 to 3.6/1.5 to 1.8 ms; flip angle 60°; and slice thickness 8 mm. Fifteen minutes after administering intravenous gadodiamide (0.2 mmol/kg, Omniscan; GE Healthcare, Waukesha, Wisconsin), an inversion recovery-prepared T1-weighted gradient-echo sequence was used for LGE-MRI in the same planes as cine-MRI ( Figure 2 ). The LGE-MRI parameters were as follows: field of view 350 to 400 mm; repetition time/echo time 4.5 to 4.6/1.3 to 1.5 ms; flip angle 15°; inversion time 200 to 300 ms; and slice thickness 8 mm. The inversion time was adjusted to nullify the signal of the normal myocardium.
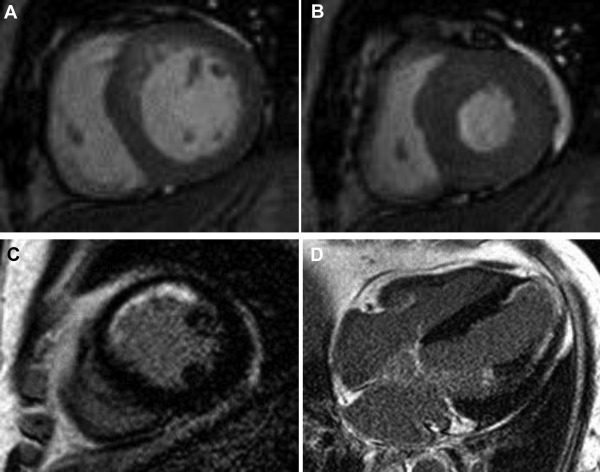
Imaging data were analyzed using a commercial postprocessing workstation (Mass; Medis, Leiden, The Netherlands). Endocardial and epicardial contours were prescribed manually on short-axis cine-MRI of the left ventricle at end-diastole and end-systole to obtain the LV volumes, mass, and LVEF. An LVEF <50% indicated LV systolic dysfunction. Wall motion abnormality (WMA) on cine-MRI and LGE on contrast-enhanced MRI were assessed using the 16-segment model by the consensus of 2 observers blinded to the patient history and clinical outcome. The WMA was graded visually on a 3-level Likert scale (normal contraction = 0; hypokinesia = 1; akinesia = 2). The WMA score was the sum of the WMA grade of each of the 16 segments. For the semiquantitative analysis of the LGE, the maximal transmural extent of LGE was determined on a 5-point Likert scale (absent = 0; 1% to 25% = 1; 26% to 50% = 2; 51% to 75% = 3; 76% to 100% = 4). The LGE score was the sum of the transmurality scores of the 16 segments. We also measured the LGE mass using the full width at half-maximum technique. The extent of LGE was expressed as the percentage of the total LV mass. For statistical analysis, the LGE score and extent were divided into 3 categories: 0, 1 to 10, and >10 and 0%, 1% to 10%, and >10%, respectively.
Clinical information was obtained from medical records and telephone interviews. Adverse cardiac events included (1) cardiac death, (2) progressive heart failure requiring hospitalization, (3) new acute myocardial infarction, and (4) unstable angina requiring hospitalization.
All statistical analyses were performed using SPSS version 22 software (IBM Corporation, Somers, New York). Continuous variables are expressed as the mean ± standard deviation and categorical variables as the count and percentage. Continuous variables were compared using the Student t test or Wilcoxon rank-sum test, and categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. A 2-sided value of p <0.05 was considered statistically significant. The associations of clinical and MRI variables with adverse cardiac events were investigated with the Cox proportional hazard model using univariate and multivariate procedures to estimate the risk of a given variable, as expressed by hazard ratios with the corresponding 95% confidence intervals. Kaplan–Meier curves were used to estimate the distribution of time to adverse cardiac events, based on an LVEF of 50% and the presence of LGE. A 3-year landmark analysis of the primary end point was also performed for patients surviving beyond 3 years after CABG. Between-group differences were evaluated with log-rank statistics.
Results
One hundred forty-six patients were included in the final analysis ( Table 1 ). Most patients had 3-vessel disease (84%) and underwent triple (34%) or quadruple (48%) bypass surgery ( Supplementary Table 1 ). Patients with LVEF <50% were more likely to have WMA or LGE and a history of hyperlipidemia, and they had significantly greater LV volume and mass indexes. Furthermore, patients with LGE were more likely to be men and have a history of previous revascularization, and they had significantly more WMA and lower LVEF ( Table 1 ).
| Characteristics | All Patients (n = 146) | Left Ventricular Ejection Fraction | Late Gadolinium Enhancement | ||||
|---|---|---|---|---|---|---|---|
| <50% (n = 53) | ≥50% (n = 93) | P Value | Presence (n = 93) | Absence (n = 53) | P Value | ||
| Clinical characteristics | |||||||
| Age (years) | 64 ± 9 | 64 ± 9 | 64 ± 9 | 0.793 | 64 ± 9 | 65 ± 9 | 0.333 |
| Men | 105 (72%) | 40 (76%) | 65 (70%) | 0.471 | 73 (79%) | 32 (60%) | 0.019 |
| Body mass index (kg/m 2 ) | 25 ± 3 | 25 ± 3 | 25 ± 3 | 0.830 | 25 ± 3 | 24 ± 34 | 0.272 |
| Hypertension | 68 (47%) | 24 (45%) | 44 (47%) | 0.813 | 45 (48%) | 23 (43%) | 0.561 |
| Diabetes mellitus | 80 (55%) | 29 (55%) | 51 (55%) | 0.989 | 51 (55%) | 29 (55%) | 0.989 |
| Hyperlipidemia | 47 (32%) | 24 (45%) | 23 (25%) | 0.011 | 34 (37%) | 13 (25%) | 0.135 |
| Smoker | 64 (44%) | 24 (45%) | 40 (43%) | 0.790 | 43 (46%) | 21 (40%) | 0.439 |
| Family history of coronary artery disease | 9 (6%) | 2 (4%) | 7 (8%) | 0.365 | 6 (7%) | 3 (6%) | 0.064 |
| Previous revascularization | 12 (8%) | 5 (9%) | 7 (8%) | 0.687 | 11 (12%) | 1 (2%) | 0.035 |
| Number of vessel involvement | 0.586 | 0.003 | |||||
| 1-vessel disease | 6 (4%) | 3 (6%) | 3 (3%) | 2 (2%) | 4 (8%) | ||
| 2-vessel disease | 18 (12%) | 5 (28%) | 13 (14%) | 6 (7%) | 12 (23%) | ||
| 3-vessel disease | 122 (84%) | 45 (85%) | 77 (83%) | 85 (91%) | 37 (70%) | ||
| Cardiac magnetic resonance imaging characteristics | |||||||
| Left ventricular mass index (g/m 2 ) | 47 ± 17 | 53 ± 20 | 44 ± 14 | 0.004 | 49 ± 15 | 45 ± 20 | 0.125 |
| Left ventricular end-diastolic volume index (mL/m 2 ) | 71 ± 31 | 93 ± 39 | 59 ± 14 | <0.001 | 72 ± 25 | 70 ± 40 | 0.787 |
| Left ventricular end-systolic volume index (mL/m 2 ) | 36 ± 26 | 60 ± 29 | 22 ± 8 | <0.001 | 38 ± 24 | 31 ± 30 | 0.111 |
| Left ventricular ejection fraction (%) | 54 ± 16 | 36 ± 11 | 64 ± 8 | <0.001 | 50 ± 16 | 60 ± 15 | <0.001 |
| Left ventricular dysfunction | 53 (36%) | 53 (100%) | 0 (0%) | – | 43 (46%) | 10 (19%) | 0.001 |
| Wall motion abnormality score | 5.1 ± 4.8 | 9.5 ± 4.7 | 2.6 ± 2.6 | <0.001 | 6.3 ± 4.6 | 3.1 ± 4.6 | <0.001 |
| Presence of late gadolinium enhancement | 93 (64%) | 43 (81%) | 50 (54%) | 0.001 | 93 (100%) | 0 (0%) | – |
| Late gadolinium enhancement score | 7.2 ± 8.2 | 12.7 ± 9.5 | 4.1 ± 5.3 | <0.001 | 11.3 ± 7.7 | 0 | – |
| Late gadolinium enhancement extent (%) | 5.3 ± 6.4 | 9.6 ± 7.8 | 2.8 ± 4.0 | <0.001 | 8.3 ± 6.4 | 0 | – |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


