At the time of hospital discharge, patients admitted with acute coronary syndrome (ACS) leave the closely monitored and protective hospital environment and are sent home, often with a sense of fear and being overwhelmed, taking 4 to 5 new medications and holding a list of both prohibited and encouraged activities. The postdischarge period is a critical time for recovery and rehabilitation. Yet there remain many unanswered questions and clinical dilemmas regarding the optimal treatment strategies. In this review, we cover 4 areas of controversy or underutilization: duration of dual antiplatelet therapy (DAPT), medicine adherence, risk stratification for sudden cardiac death (SCD), and cardiac rehabilitation. In each section, we review current guidelines, review the relevant literature, highlight areas of uncertainty, and look forward toward ongoing research.
Controversy on Optimal DAPT Duration After ACS
Current guideline recommendations on duration of DAPT
A large body of evidence supports that patients who survived an ACS should be treated with DAPT for 12 months regardless of the treatment strategy during the acute phase (i.e., medical management vs percutaneous coronary intervention [PCI] with stent vs coronary artery bypass graft [CABG]). For patients with unstable angina/non–ST elevation myocardial infarction (UA/NSTEMI), the current American College of Cardiology Foundation/American Heart Association 2012 focused update recommends that aspirin should be continued indefinitely (class I recommendation, level of evidence: A). The guideline also recommends that a P2Y 12 receptor inhibitor be continued for up to 1 year for patients who did not receive stents or received bare-metal stents (BMSs) (class I recommendation, level of evidence: B). For patients who received drug-eluting stents (DESs), the guideline recommends that a P2Y 12 receptor inhibitor be continued for at least 12 months (class I recommendation, level of evidence: B).
With regard to patients who presented with ST elevation myocardial infarction (STEMI), the current guideline published in 2013 recommends continuing aspirin indefinitely. The guideline recommends continuing DAPT for at least 1 year if the patient received a DES and for at least 1 month (up to 1 year) if a BMS was chosen ( Table 1 ).
| Maintenance doses and duration of therapy | COE | LOE |
|---|---|---|
| DES placed: continue therapy for 1 year with: | ||
| Clopidogrel: 75 mg daily | I | B |
| Prasugrel: 10 mg daily | I | B |
| Ticagrelor: 90 mg twice daily ∗ | I | B |
| BMS † placed: Continue therapy for 1 year with: | ||
| Clopidogrel: 75 mg daily | I | B |
| Prasugrel: 10 mg daily | I | B |
| Ticagrelor: 90 mg twice daily ∗ | I | B |
| DES placed: | ||
| Clopidogrel, prasugrel, or ticagrelor ∗ continued beyond 1 year | IIb | C |
| Patients with STEMI with prior stroke or TIA: prasugrel | III: Harm | B |
∗ The recommended maintenance dose of aspirin to be used with ticagrelor is 81 mg daily.
† Balloon angioplasty without stent placement may be used in selected patients. It might be reasonable to provide P2Y 12 inhibitor therapy to patients with STEMI undergoing balloon angioplasty alone according to the recommendations listed for BMS (LOE: C).
Review of data on continuation of DAPT for >12 months
One of the major unanswered clinical questions is whether continuation of DAPT for >12 months would be beneficial for patients who experienced a myocardial infarction (MI). The current guideline for UA/NSTEMI states that “continuation of a P2Y 12 receptor inhibitor beyond 12 months may be considered in patients after DES placement” (class IIb recommendation, level of evidence: C). Data from an observational study suggested that patients who received DES and tolerated DAPT with aspirin and clopidogrel for 12 months without adverse cardiac events experienced fewer deaths and the composite of death or MI if they continued DAPT for >12 months. No such benefit was observed among patients who were treated with a BMS. In contrast, when data from 2 randomized controlled trials were combined, no difference was observed from prolonged DAPT with a median follow-up period of 19.2 months. None of these studies were designed or powered to detect late stent thrombosis or bleeding events.
Development of newer antiplatelet agents and uncertainty in the optimal duration of DAPT
Another important factor that should be taken into consideration when determining the optimal duration of DAPT after an ACS event is that there have been 2 newer antiplatelet agents introduced that show better efficacy compared with clopidogrel in phase 3 clinical trials: ticagrelor and prasugrel.
Review of the TRITON-TIMI 38 and PLATO studies
Prasugrel is a thienopyridine with faster onset of action and increased inhibition of platelet aggregation than clopidogrel. The TRial to Assess Improvement in Therapeutic Outcomes in Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis in Myocardial Infarction (TRITON-TIMI 38) trial was a randomized controlled trial with 13,608 patients who presented with ACS and were scheduled to undergo PCI. Patients were randomized to prasugrel (60 mg loading dose followed by 10 mg/day) or clopidogrel (300 mg loading dose, 75 mg/day) and followed for up to 15 months. The primary efficacy end point was a composite of cardiovascular death, MI, or stroke. The incidence of the primary efficacy end point was significantly lower in the prasugrel group than in the clopidogrel group (9.9% vs 12.1%, respectively, p <0.001) at the cost of increased rates of non–CABG-related bleeding. Furthermore, the risk of bleeding was excessive among patients with a history of stroke or transient ischemic attack.
Ticagrelor is a nonthienopyridine and reversibly inhibits the P2Y 12 receptor. Ticagrelor demonstrates more potent and rapid onset of platelet inhibition compared with clopidogrel. The PLATlet inhibition and patient Outcomes (PLATO) trial randomized a broad cross section of 18,624 patients with ACS to receive ticagrelor (180 mg loading dose, 90 mg twice daily) or clopidogrel (300 mg loading dose, 75 mg/day) for 12 months. The primary end point of the composite of cardiovascular death, MI, or stroke occurred less frequently in the ticagrelor group compared with the clopidogrel group (9.8% vs 11.7%, respectively; p <0.001). Ticagrelor also reduced cardiovascular and overall mortality, but at the cost of increased non-CABG bleeding.
Both trials demonstrated the superiority of more potent adenosine diphosphate inhibition compared with clopidogrel; however, because both trials continued therapy for approximately 1 year, they were not able to provide greater insight into the benefit of prolonged DAPT.
Recent trials
There are 2 recent trials that will provide important information regarding the optimal DAPT duration after ACS: the Dual Antiplatelet Therapy (DAPT) study and the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin (PEGASUS) study.
DAPT study
The DAPT study is an international, multicenter, randomized, double-blind, placebo-controlled trial that plans to enroll 15,245 patients treated with DES and 5,400 treated with BMS. If no major adverse cardiovascular and cerebrovascular event (all deaths, MI, and stroke) or Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) moderate or severe bleeding is observed after receiving DAPT for 12 months, the patients will be randomized to receive either 18 additional months of thienopyridine (clopidogrel or prasugrel) or placebo on a background therapy with aspirin. The coprimary end points are major adverse cardiovascular and cerebrovascular event and stent thrombosis, and the primary safety end point is GUSTO moderate or severe bleeding.
PEGASUS study
Another clinical trial that may give further insights into this issue is the PEGASUS study. This study is randomizing 21,000 patients who had an MI within 1 to 3 years and have at least 1 additional risk factor to either ticagrelor 90 or 60 mg twice daily or placebo twice daily on top of the background therapy with aspirin. The primary end point is a composite of cardiovascular death, nonfatal MI, or nonfatal stroke.
These 2 trials may provide more reliable information as to when to stop DAPT after having ACS.
Underutilization of Medications Recommended by the Guidelines
Despite a broad evidence base for postdischarge medication recommendations, underutilization remains a significant limitation to achieving high levels of guideline-recommended care. In this section, we will first review the current recommendations from the guidelines in terms of medication prescriptions. Recent data will be reviewed on prescription rate on discharge, compliance within 7 days after discharge, and adherence to medications in the longer term.
Review of current guideline recommendations for discharge prescriptions
As mentioned previously, current guidelines for STEMI and UA/NSTEMI recommend DAPT with aspirin and a P2Y 12 receptor inhibitor for up to 12 months if tolerated. Beta blockers, for both STEMI and UA/NSTEMI, should be continued at discharge if there are no contraindications (class I recommendation, level of evidence: B). Angiotensin-converting enzyme inhibitors (ACE-I), or angiotensin receptor blockers (ARB) if ACE-I is not tolerated, are recommended for patients with UA/NSTEMI with heart failure, left ventricular (LV) dysfunction (LV ejection fraction [LVEF] <0.40), hypertension, or diabetes mellitus unless contraindicated. For patients with STEMI, ACE-I or ARB should be started within 24 hours if anterior location, heart failure, or LVEF ≤40% is present (class I recommendation, level of evidence: A). Regarding aldosterone antagonists, the guideline for STEMI states that an aldosterone antagonist should be given to patients with STEMI and no contraindications who are already receiving an ACE-I and a β blocker and who have ejection fraction ≤0.40 and either symptomatic heart failure or diabetes mellitus (class I recommendation, level of evidence: B). For patients with UA/NSTEMI, long-term aldosterone receptor blockade should be prescribed for patients without significant renal dysfunction or hyperkalemia who are already receiving therapeutic doses of an ACE-I, have an LVEF ≤40%, and have either symptomatic heart failure or diabetes mellitus (class I recommendation, level of evidence: A). The guidelines also recommend the use of high-intensity statins for patients with STEMI (level of evidence: B) and the use of statins for patients with UA/NSTEMI (level of evidence: A).
Prescription rate at discharge
The prescription rate of aspirin, β blockers, ACE-I/ARB, and statins on discharge of patients is generally high, ranging from 90% to 98% according to the recently published data from 72,352 patients admitted for acute MI in the United States. However, the prescription rate for clopidogrel was only 73.2% (85.6% for STEMI and 67.0% for NSTEMI; Table 2 ).
| Description (%) | Overall (n=72 352) | STEMI (n=23 386) | NSTEMI (n=48 966) |
|---|---|---|---|
| Performance measure (eligible patients only ∗ ) | |||
| Aspirin within 24 h of arrival | 44 854 (97.5) | 14 806 (98.5) | 30 048 (97.1) |
| Discharge on aspirin | 56 410 (97.7) | 19 388 (98.5) | 37 022 (97.3) |
| ACE inhibitor or ARB on discharge for LV systolic dysfunction | 10 200 (92.5) | 3803 (95.0) | 6397 (91.1) |
| Discharge on β-blockers | 55 203 (97.3) | 18 640 (98.2) | 36 563 (97.0) |
| Lipid-lowering therapy for LDL >100 mg/dL | 14 804 (93.5) | 6542 (96.8) | 8262 (91.0) |
| Smokers who receive smoking cessation counseling | 19 893 (97.7) | 8610 (97.9) | 11 283 (97.5) |
| Composite performance: compliance with all applicable performance measures | 62 214 (92.1) | 20 638 (94.3) | 41 576 (91.1) |
| Quality measure (eligible patients only ∗ ) | |||
| β-blockers within 24 h of arrival | 37 304 (91.8) | 12 133 (93.9) | 25 171 (90.8) |
| Discharge on ACE inhibitors or ARB | 43 908 (80.1) | 15 956 (85.3) | 27 952 (77.4) |
| Discharge on clopidogrel | 40 185 (73.2) | 15 855 (85.6) | 24 330 (67.0) |
| Lipid-lowering therapy at discharge | 54 045 (90.2) | 18 969 (94.8) | 35 076 (88.0) |
∗ Eligible patients are those who lack contraindications to a particular measure.
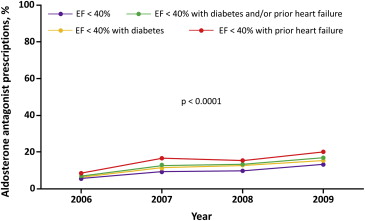
Of eligible patients, only 9.1% received an aldosterone antagonist at hospital discharge. Recent data show that the prescription rate of aldosterone antagonist for eligible patients after an MI event is gradually increasing, as shown in Figure 1 ; however, it still remains low at 10% to 20% depending on patient population and characteristics.
Adherence rate at 7 days
Patient adherence to guideline-recommended medication remains challenging. Data from Ontario, Canada, reported that only 77% of patients filled all their discharge prescriptions for cardiac medications by 7 days after discharge ( Table 3 ). (This study did not assess prescription filling rate of aspirin, as it was also available over the counter in Ontario.) More importantly, patients who did not fill any of the prescribed medications after an acute MI event had a higher mortality with the odds ratio at 1.80 (95% confidence interval [CI] 1.35 to 2.42, p <0.0001). Similarly, patients who filled only some of the prescribed medications also carried a higher mortality rate (odds ratio 1.44, 95% CI 1.15 to 1.79, p = 0.001).
| Discharge prescriptions dispensed by day indicated, % | |||||
|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 30 | Day 90 | Day 120 | |
| All medications | 73.0 | 73.8 | 75.2 | 77.8 | 78.6 |
| Noncardiac medications | 27.4 | 28.9 | 30.6 | 34.1 | 34.6 |
| Cardiac medications | 76.8 | 77.6 | 79.0 | 81.4 | 82.3 |
| ACE inhibitors | 92.0 | 92.6 | 94.1 | 95.6 | 96.2 |
| Antiplatelets ∗ | 48.6 | 49.2 | 50.6 | 54.2 | 55.7 |
| β-Blockers | 87.8 | 88.4 | 89.4 | 91.4 | 92.0 |
| Calcium channel blockers | 87.4 | 88.4 | 89.9 | 92.4 | 92.7 |
| Lipid-lowering agents † | 88.0 | 88.4 | 89.7 | 93.0 | 94.0 |
| Nitrates | 86.7 | 87.9 | 89.6 | 91.6 | 92.1 |
| Statins | 88.9 | 89.3 | 90.6 | 93.8 | 94.8 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


