Radiotherapy for breast cancer may expose heart and vessels to late radiation-induced complications. Although recent technical progress in radiation therapy (RT) has been associated with drastic reduction in cardiovascular (CV) mortality, the prolonged life expectancy of patients with cancer requires CV evaluation for many years. The aim of our study was to evaluate local changes in vascular and cardiac function because of previous breast RT. We enrolled 43 patients treated with RT 15 years ago for breast cancer. CV risk factors and atherosclerotic carotid damage were investigated in all women. We divided patients into 2 groups: R (n = 25) treated to right breast and L (n = 18) to left breast. All subjects were submitted to standard echocardiography and functional arteries evaluation by carotid-radial pulse-wave velocity (crPWV; Complior) and AIx (Sphygmocor; Atcor Medical). Global mean age was 69.5 ± 8 years old. CV risk factors were equally allocated in 2 groups. No patients had history of cardiac or artery disease. R had a significantly increased crPWV (9.9 ± 1.4 vs 8.9 ± 1.1, p = 0.001) on right arm compared with left arm, and in L group, crPWV was similarly higher on the left arm than on right arm (9.6 ± 1.5 vs 8.9 ± 1.4, p = 0.011). AIx was significantly increased in the ipsilateral arm only in L (32.1 ± 7.6 vs 28.3 ± 6.8, p = 0.05). Central blood pressure estimation was not different in the right and left arms. No correlations were found with hormone therapy or chemotherapy. Our data show a local arterial stiffening because of radiation that can be involved in increased CV risk in breast cancer–treated patients.
The cardiovascular (CV) effects of cancer therapy are critically important to the overall health of cancer survivors as this population is growing and is recognized to have an increased incidence of hypertension, valvular disease, and cardiomyopathy compared with general population. Radiation therapy (RT) has been associated with an increased risk of coronary disease because during RT both malignant and normal cells (and among this vascular and endothelial cells) inside the treatment volume are exposed to ionizing effects and, thus, to potentially relevant damage. Vascular damage is believed to be secondary to damage to the microvasculature that results in endothelial dysfunction, inflammation, and oxidative stress that could determine arterial stiffening and increase the probability to develop atherosclerotic plaque. To date, there are no conclusive information about the effect of RT on arterial stiffness, that is, a recognized predictor of CV morbidity and mortality. Therefore, the aim of our study was to significantly expand information on CV effects of RT, both in terms of subclinical vessel organ damage and cardiac alterations, by collecting data on an array of sensitive markers of both cardiac and vascular function in a cohort of women with breast cancer treated with RT over 15 years ago.
Methods
We investigated 43 female subjects diagnosed and treated with surgery for breast cancer in 1997. The inclusion criteria were a surgical treatment with quadrantectomy and axillary dissection and an adjuvant radiation treatment to the breast to a standard total dose of 60 Gy in 30 fractions (50 Gy to the whole breast followed by 10 Gy to the surgical bed); 25 women were treated to the right breast and 18 to the left breast. A further criterion was to be followed by our oncologic department and, after 15 years, recalled.
Carotid-femoral PWV (cfPWV) and carotid-radial PWV (crPWV) were measured by an automatic device (Complior, Artech, France) capable of assessing the rapid upstroke of the foot of arterial pulsewave. With the patient supine, we measured separately both cfPWV and crPWV. For the assessment of cfPWV, we recorded blood pressure (BP) waveforms from common carotid to femoral artery. The 80% carotid-femoral artery distance was measured by a rigid rule, according to current international guidelines, and the cfPWV was calculated as the ratio between this value and the pulse transit time. For crPWV, the BP waveforms were recorded at right side from right common carotid to right radial artery and at left side from left common carotid to left radial artery. The carotid-radial distance was similarly calculated by a rigid rule and the crPWV was calculated as the ratio between this value and the pulse transit time. Pulse transit time was always determined from the average of 10 consecutive cardiac beats to collect data on a complete respiratory cycle, and the mean of the 2 complete cardiac beat was used for analysis. In our laboratory, the intra-session within- and between-operator variability of the cfPWV and crPWV values, expressed as coefficient of variation of the mean, are 3% and 4%, respectively. The within-operator variability between sessions is 4%. Aortic stiffness was defined as a cfPWV measurement >10 m/s accordingly to current guidelines.
For measurement of carotid intima-media thickness (CIMT), longitudinal images of the far wall of the common carotid artery, of the bifurcation (bulb), and of the internal carotid artery were acquired. CIMT was taken as the distance from the leading edge of the first echogenic line to the leading edge of the second echogenic line and will be measured using an automatic real-time system (Esaote MyLab Twice). Averaged values of the CIMT of each of the 6 segments (3 on each side) will be used as the CIMT values for each subject. An IMT >0.9 mm was defined as the presence of subclinical organ damage and carotid plaque was defined as the presence of an IMT >1.2 mm in the common carotid, bulb, or internal carotid artery.
PWA and central BP were determined through a Millar piezoresistive pressure transducer connected to a device (Sphygmocor; Atcor Medical, Sydney, Australia) that allows to obtain the arterial waveform from the applanation of the radial artery at the site of its maximum pulsation. Calculation of central systolic, diastolic, and pulse pressures was provided by a validated transfer function, following the software calibration on values obtained sphygmomanometrically at the brachial artery site. Collected data included the augmentation index (Aix), that is, the supplementary increase in systolic BP determined by the reflected pressure waves, according to the following formula: Aix = (AP/PP) × 100, where AP is the pressure difference between the shoulder of the wave and the peak systolic pressure and PP the pulse pressure. Because of their dependence on heart rate, data were normalized for a value of 75 beats/min (Aix@HR75). The Aix measure was performed both at right and left radial artery in each patients. The average of 2 valid recordings was used in our analysis. In our laboratory, the intra-session within- and between-operator variability of the Aix values, expressed as coefficient of variation of the mean, are 3% and 5%, respectively. The within-operator variability between sessions is 4%.
Echocardiographic examinations were performed through a commercially available device (GE Vivid E9; General Electric Ultrasound, Milwaukee, WI), equipped with 3.5 MHz tissue harmonic imaging probe according to the recommendations of the American Society and the European Association of Echocardiography: B-mode measurements of interventricular septum, left ventricular (LV) posterior wall thickness, and end-diastolic and end-systolic diameters were obtained in accordance with current guidelines. LV mass was calculated by the Devereux’s formula and end-diastolic and end-systolic LV volume and ejection fraction by the biplane Simpson’s method and regional function by the Wall Motion Score Index. Pulse-wave Tissue Doppler Imaging was performed to assess myocardial systolic function by systolic wave velocity (S) measured in the apical 4-chamber views; S wave is the peak contraction velocity of basal segment of septum and lateral wall in left ventricle and is measured in m/s. Measurements included diastolic function based on left atrial volume, pulse-wave Doppler mitral inflow, and pulse-wave tissue Doppler imaging in the apical 4-chamber views to acquire septal and lateral mitral annular velocities. In our laboratory, the intra-session and inter-session within- and between-operator variability of the standard echocardiographic measurements, expressed as coefficient of variation of the mean, are between 3% and 7%. LV hypertrophy was defined by LV mass index ≥110 g/m 2 for men and ≥95 g/m 2 in women.
All women responding to inclusion criteria, previously followed up by RT colleagues, and not affected by major arrhythmias, actual neoplastic, or other major systemic pathologies were asked to come to the outpatient clinic of our hospital in the afternoon. They were submitted to a history and physical examination to note patient’s status and pharmacologic therapy. The history also aimed to investigate the presence of CV risk factors and cardiac events happened after the oncologic treatment. After 10 minutes of rest in a quiet room in which the temperature was maintained at 22°C, brachial BP was measured 3 times using a standard sphygmomanometer and keeping the subject in the sitting position. Well-controlled BP was defined as systolic BP of <140 mm Hg and/or diastolic BP of <90 mm Hg. BP measurements were followed by vascular and cardiac evaluations. The subjects were placed in the supine position, and the carotid eco-color Doppler was done to judge the presence of plaques or pathologic IMT. Assessment of the Aix and the crPWV were performed both at right and left sides. Then all women were subjected to the standard cardiac echocardiography. Data analysis was performed using SPSS software (version 20) for data management and statistical analyses. Continuous variables were expressed as mean ± SD and dichotomous data as percentages. We compared in all subjects the vascular data (PWV and Aix) registered in the right side with those obtained in the left side. The comparisons were made by ANOVA and the 2-tailed t test. A p value <0.05 was taken as the level of statistical significance.
Patients consented in writing to participate in the study after being informed of its nature and purpose. The study protocol complies with the Declaration of Helsinki (as revised in 2004) and was approved by the Ethic Committee of the Niguarda Ca’ Granda Hospital.
Results
Table 1 lists the clinical characteristics of our population, distinguishing women irradiated on the right side (right RT) and on the left side (left RT). CV risk factors were equally allocated in 2 groups; 25 subjects (15 women in right breast group and 10 in left one) had arterial hypertension. Only 6 patients were affected by diabetes mellitus type II in oral treatment (3 in each group). Dyslipidemia, as defined by the European Society of Cardiology/European Society of Cardiology guidelines, was disclosed in 40% of subjects in both groups. None of our patients had history of cardiac artery disease. In our population, we observed a low prevalence of active smokers; 16 women had an increase of IMT or carotid plaques at ultrasonography. Mean cfPWV and LV mass index were in the normal range. Table 1 also lists the rate of patients treated with chemotherapy (60%) and hormone therapy (52% in right group vs 61% in left one) in the meantime of RT. No statistically significant difference was seen in systemic target organ damage and other variables between the L and R groups.
| Variables | All Patients (n = 43) | Right RT (n = 25) | Left RT (n = 18) |
|---|---|---|---|
| Age (years) | 69.5 ± 8 | 70.7 ± 7.8 | 67.8 ± 8.4 |
| Previous chemotherapy | 26 (60%) | 15 (60%) | 11 (61%) |
| Previous Hormonal therapy | 24 (56%) | 13 (52%) | 11 (61%) |
| BMI (kg/m 2 ) | 25.4 ± 4.0 | 25.4 ± 4.1 | 25.3 ± 4.1 |
| Hypertension | 25 (58%) | 15 (60%) | 10 (56%) |
| Dyslipidemia | 17 (40%) | 10 (40%) | 7 (39%) |
| Diabetes mellitus | 6 (14%) | 3 (12%) | 3 (17%) |
| Current/ex smoker | 3/10 (7%/23%) | 2/7 (8%/28%) | 1/3 (7%/17%) |
| Coronary artery disease | 0 | 0 | 0 |
| Brachial blood pressure (mmHg) | 134 ± 14 / 76.7 ± 9 | 134.5 ± 9.8 / 77.5 ± 8.7 | 133.5 ± 18.2 / 75.9 ± 9.7 |
| Heart Rate (beat/min) | 69.6 ± 9.4 | 68.8 ± 9.8 | 70.6 ± 9 |
| Carotid-femoral Pulse Wave Velocity (m/sec) | 9.8 ± 2.7 | 8.8 ± 1 | 10.4 ± 3.5 |
| Carotid intima-media thickness ≥ 0.9μ or carotid plaques | 16 (37%) | 10 (40%) | 6 (33%) |
| Left ventricular mass index (g/m 2 ) | 85.8 ± 20.8 | 86.4 ± 22.6 | 84.9 ± 18.4 |
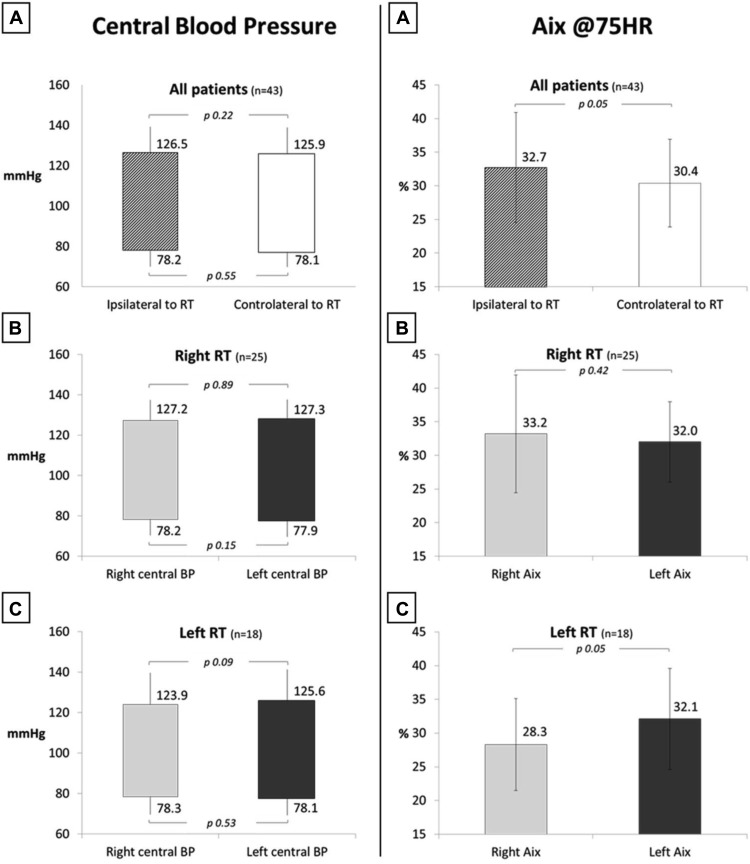
Brachial BP was well controlled in all patients with or without a history of hypertension ( Table 1 ). Figure 1 shows that women submitted to right and left irradiations have similar central BP values measured at right and left sides. Therefore, no differences in aortic pressure are registered in patients submitted to RT.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


