Lobectomy: Robotic
Tyler Grenda
Jules Lin
DEFINITION
Robotic-assisted thoracoscopic lobectomy is defined as an anatomic lobectomy performed with the assistance of robotic technology in which bronchial and vascular ligation is performed with lymph node dissection or sampling through several small incisions while avoiding spreading of the ribs. The technique is a minimally invasive alternative to thoracoscopic lobectomy and must be performed with the same oncologic principles as an open lobectomy including vascular ligation, resection with negative margins, and appropriate lymph node dissection.
PATIENT HISTORY AND PHYSICAL FINDINGS
A detailed history and physical must be performed including past medical and surgical history, allergies, medications, social, and family history.
A detailed social history is important to determine the patient’s previous history of tobacco use as well as any chemical or asbestos exposures. Smoking cessation for 4 weeks preoperatively should be strongly encouraged prior to surgery.
The patient’s current functional status and exercise tolerance must also be assessed to determine their fitness for surgery.
A complete physical examination should be performed with auscultation of the heart and lungs and evaluation for any cervical or supraclavicular lymphadenopathy or peripheral edema. Any abnormal findings on physical examination should be evaluated prior to surgery.
Routine laboratory studies including a complete blood count and basic chemistry panel should be included as part of the preoperative evaluation.
IMAGING AND OTHER DIAGNOSTIC STUDIES
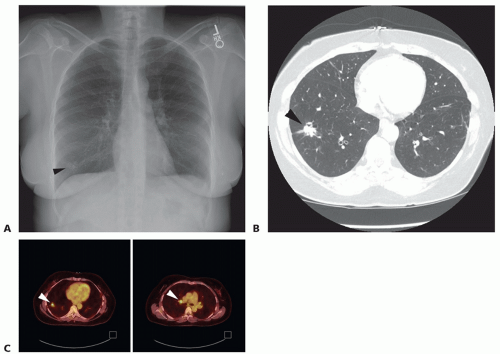
Patients generally present with abnormalities on chest radiograph (FIG 1A) or chest computed tomography (CT) (FIG 1B). The lung nodule should be compared to previous imaging when available. Nodules that have been stable for more than 2 years are generally benign. Indeterminate lesions less than 1 cm in size should be followed with serial imaging according to the recommendations of the Fleischner Society.1
A chest CT (FIG 1B) should be obtained in all patients with a suspicious lung nodule on chest x-ray (CXR)2,3 to evaluate the size and characteristics of the lesion; any potential involvement of the chest wall, vessels, airway, and mediastinum; additional pulmonary nodules; and hilar or mediastinal lymphadenopathy. The upper abdomen should be included to evaluate the liver and adrenal glands for any metastatic disease.
A positron emission tomography (PET) scan (FIG 1C) is a functional study that evaluates the metabolic activity of the pulmonary lesion and areas of uptake that are suspicious for regional nodal or distant metastatic disease and should be obtained in patients suspected of having a non-small cell lung carcinoma.3 Sampling of any abnormal lymph nodes
should be performed prior to lung resection by endobronchial ultrasound (EBUS) or mediastinoscopy.
Due to the low morbidity and mortality of a thoracoscopic wedge resection, for nodules that are highly suspicious for lung carcinoma on PET or serial imaging, our preference is to perform a video-assisted thoracic surgery (VATS) wedge resection for a tissue diagnosis. CT or super dimensional needle biopsies are performed when the diagnosis is less clear or for central lesions that would require a lobectomy for diagnosis alone.
Once the cancer diagnosis is confirmed, an anatomic lobectomy and mediastinal lymph node dissection for complete oncologic resection and staging is performed in patients who are surgical candidates.4
As screening chest CTs identify smaller lesions, preoperative localization with methylene blue or coil/wire placement by CT guidance or super dimensional bronchoscopy may be useful in identifying small (<5 mm) or ground glass nodules (<1 cm) and nodules that are deep to the pleural surface intraoperatively.
SURGICAL MANAGEMENT
Preoperative Planning
All patients undergoing lung resection should undergo preoperative pulmonary function testing (PFT) to determine if they have adequate pulmonary reserve.5 Preoperative cardiac evaluation should be performed in patients with significant cardiovascular risk factors or symptoms.
Patients with a preoperative forced expiratory volume in 1 second (FEV1) of more than 60% predicted and diffusing capacity of lung for carbon monoxide (Dlco) of more than 50% predicted are candidates for lobectomy. Patients not meeting these criteria should undergo further testing with a quantitative ventilation perfusion scan to determine the postoperative predicted pulmonary function with a minimum postoperative value of 40% predicted.
Cardiopulmonary exercise testing can be helpful in patients whose symptoms do not correlate with the severity of their pulmonary function results.
For patients who are not candidates for an anatomic lobectomy, alternatives include a sublobar resection such as a segmentectomy or wedge resection, stereotactic body radiation therapy (SBRT), radiofrequency ablation (RFA), or definitive chemoradiation. These patients should be discussed in a multidisciplinary setting.
In the preoperative area, the history and physical should be reviewed and consent should be obtained from the patient. The operative side should be marked.
Once in the operating room (OR), a flexible bronchoscopy is performed to verify airway anatomy and the location of any endobronchial lesions.
A left-sided double lumen endotracheal tube is generally preferable to a bronchial blocker in achieving single-lung ventilation, especially on the right where the mainstem bronchus is shorter.
It is important to recognize the lack of tactile feedback when using the robot. Practice in the robotic simulator prior to the case is useful.
The OR team must be familiar with emergency de-docking, and a sponge stick should be immediately available in case of bleeding.
The dual console is useful when working with residents and allows both surgeons to switch instruments as needed. Pressing the camera pedal stops all the arms from moving, increasing safety.
Positioning
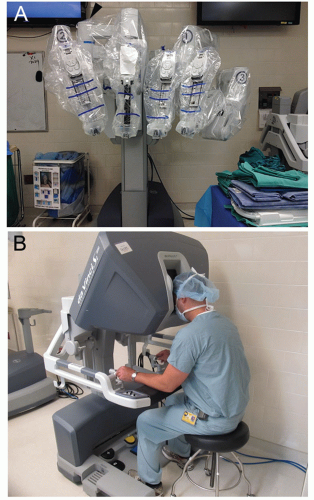
The robot is draped sterilely (FIG 2A). The surgeon operates with arms 1 and 2 while arm 3 is used for retraction. The joint of the camera arm should be placed opposite of arm 3 to minimize bumping of the robotic arms.
The surgeon sits at the console (FIG 2B), whereas an experienced bedside assistant changes the robotic instruments, passes the staplers, and suctions when needed.
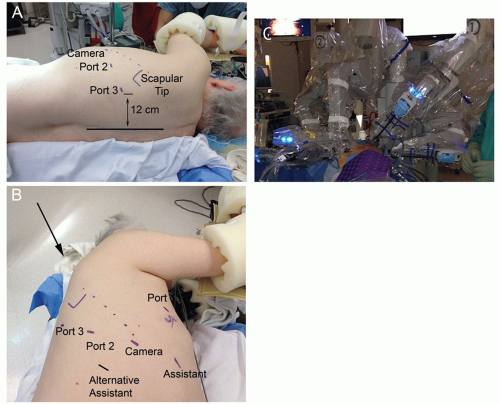
The patient should be placed in the lateral decubitus position, tilted slightly posteriorly (FIG 3A). The robotic camera is much larger than the standard thoracoscopic camera, and the bed is flexed taking care to drop the hips out of the way of the camera port. The patient should be secured to the bed, and all pressure points are padded. The arms are positioned in the neutral position in an arm holder.
The position of the endotracheal tube should be confirmed again once positioning is complete.
PLACEMENT OF INCISIONS
Port placement is important in obtaining the ideal angles for stapler placement and avoiding the robotic arms interfering with each other or the bedside assistant’s port. Our general port placement for a robotic lobectomy is shown (FIG 3B).
Port 1 is placed in the 5th intercostal space in the anterior axillary line. A 5-mm, 30-degree angled standard thoracoscope is inserted, so the remaining ports can be placed under direct visualization.
Port 2 is placed in the 7th intercostal space in the posterior axillary line. Port 3 is placed in the 7th intercostal space in the auscultatory triangle and should be placed at least 12 cm from the spine. The camera port is placed in the 7th intercostal space in the midaxillary line, so the camera will be directed in line with the major fissure.
A 12- to 15-mm assistant port is placed in the 7th intercostal space in the anterior axillary line. It is important to carefully place the assistant port so that the bedside assistant is able to pass instruments without interference from the robotic arms (FIG 3C). For a right upper or middle lobectomy, placing the assistant port more posteriorly in the 8th intercostal space offers a better stapler angle. Early in the surgeon’s experience, the 15-mm port is useful, so standard thoracoscopic instruments such as the Foerster clamp and long coronary tip suction can be used.
Carbon dioxide (CO2) insufflation to 5 mmHg can be useful for a lower lobectomy to help push the diaphragm out of the way. It is important to communicate with the anesthesiologist at the start of insufflation to watch for hypotension and adjust the CO2 insufflation as warranted.
A 10-cm access incision can be made instead of the assistant port at the beginning of the case. If CO2 insufflation is used, the access incision is made at either the assistant port or port 1 at the end of the case to remove the specimen. In the event of bleeding, a sponge stick can be quickly inserted through the access incision or the 15-mm port to hold pressure.
The robot is brought in at a 30-degree angle to the back in line with the major fissure (FIG 3B).
The robot is docked to the ports with either a bipolar Maryland or fenestrated grasper in port 1 for dissection. A ProGrasp is placed in port 2 and is used to provide counter tension as vessels and other structures are dissected free. A Caudier grasper is placed in port 3 and is used to retract and position the lung. A 30-degree, 8.5-mm camera (angled down) is used to decrease compression of the intercostal nerve.
Following port placement, a thoracoscopic exploration is performed to evaluate for any effusion, pleural lesions, or additional pulmonary nodules.
Adequate lung isolation is important, and if the lung is not adequately collapsed, suction should be applied to the appropriate lumen of the endotracheal tube.
RIGHT UPPER LOBECTOMY
The location of the nodule in the right upper lobe should be confirmed. The lung is retracted posteriorly to expose the hilum.
The dissection is performed from anterior to posterior. The anterior mediastinal pleura is opened sharply to avoid injuring the phrenic nerve using scissors or the bipolar Maryland grasper (FIG 4A).
The superior pulmonary vein branches are dissected bluntly with the bipolar fenestrated grasper. The division between the upper lobe and middle lobe venous branches must be identified (FIG 4B). The minor fissure can be followed anteriorly to ensure that the middle lobe vein is preserved. Care must be taken to avoid injury to the underlying right pulmonary artery.
The upper lobe superior venous branches are divided using a vascular stapler (Ethicon Inc, San Antonio, TX or Covidien, Mansfield, MA; beige load Endo GIA, 2.0-to 3.0-mm stapler height) through the assistant port (FIG 4C). If needed, the vessels can be encircled with a silk tie for traction or a curved-tip stapler (Covidien) can be used to assist in passage of the stapler.
The truncus anterior branch of the pulmonary artery is then dissected free and divided using a beige load stapler through the assistant port (FIG 4D).
With the lung retracted anteriorly, the posterior pleura is opened. The bifurcation between the upper lobe bronchus and the bronchus intermedius is dissected free (FIG 4E). An 11R lymph node is often removed, and care must be taken not to injure the posterior ascending pulmonary artery branch, which is just behind this lymph node.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree