Linear Left Atrial Ablation
Mélèze Hocini
Kang-Teng Lim
Prashanthan Sanders
Pierre Jaïs
Seiichiro Matsuo
Sébastien Knecht
Leonardo Arantès
Mark O’Neill
Yoshihide Takahashi
Jacques Clémenty
Michel Haïssaguerre
Over the last decade, catheter ablation has emerged from being a feasible to a realistic therapeutic option for patients with atrial fibrillation (AF). Since the initial discovery that AF can be initiated by triggers from the pulmonary veins (PV), published studies have demonstrated the efficacy of the PV isolation procedure to cure paroxysmal AF and significantly reduce AF burden. Catheter ablation targeting PV alone is ineffective for patients with longer-lasting persistent and permanent AF. The principal ablation techniques in these patients include not only PV isolation but also electrogram-guided ablation and left linear ablation. Each of these techniques has been shown to be effective, but when employed in combination are associated with improved clinical outcome in most persistent and permanent AF.
While left atrial linear ablation is indicated in the case of macroreentrant tachycardia, the respective place and order of linear lesion and ablation of left atrial electrophysiologic targets to modify LA substrate during AF is still to be studied. Combining linear lesions with PV isolation has led to increased efficacy of catheter ablation for both paroxysmal and persistent AF (1, 2, 3, 4, 5). The technical difficulty of linear lesion argues against the use of this technique early in the course of the catheter ablation procedure while the inability to achieve a complete line may be proarrhythmic.
This review describes the currently used techniques to perform and evaluate linear lesions by catheter ablation. In addition, the consequences of linear lesion are discussed in detail.
In our institution, PV isolation and ablation of the cavotricuspid isthmus are performed in all patients undergoing catheter ablation of AF. The techniques used for PV ablation involve the use of a circular catheter (Lasso, Biosense-Webster, Diamond Bar, CA) to guide ablation (6). In our experience, a power limited to 30 W with
temperature limited to 50°C, using irrigation rates of 5-20 mL/min to achieve the desired power delivery is generally sufficient. Electrical isolation of the PV is confirmed by either elimination or dissociation of PV potentials. Depending on operator preference, adjunctive modalities including 3-D electroanatomic mapping (CARTO, Biosense-Webster), Ensite NavX (St. Jude Medical, St. Paul, MN) or intracardiac echo may also be used.
temperature limited to 50°C, using irrigation rates of 5-20 mL/min to achieve the desired power delivery is generally sufficient. Electrical isolation of the PV is confirmed by either elimination or dissociation of PV potentials. Depending on operator preference, adjunctive modalities including 3-D electroanatomic mapping (CARTO, Biosense-Webster), Ensite NavX (St. Jude Medical, St. Paul, MN) or intracardiac echo may also be used.
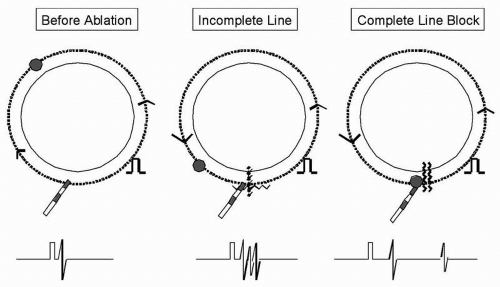
Cavotricuspid isthmus ablation is performed using a linear dragging technique. The endpoint of ablation is demonstration of bidirectional conduction block in sinus rhythm, corridor of double potentials on the line, activation detour in the opposite direction, and confirmation with pacing techniques. Assessment for cavotricuspid isthmus ablation forms the basis of complete linear ablation and is illustrated in Figure 12.1.
If AF persists after PV isolation and electrogram-guided LA ablation or if macroreentrant tachycardia develops around the mitral annulus or the right PVs, linear ablation is considered (7). Used in this context, linear ablation is performed in up to 40% of patients with paroxysmal AF and in most patients with persistent or permanent AF. While this is the approach that we have used to guide the extent of ablation, another acceptable approach in patients with paroxysmal AF would be to restrict the use of linear ablation to those with clinical recurrence after PV isolation. In addition, the relative roles of linear ablation compared with the use of electrogram-guided ablation in an individual patient may still evolve with progress of AF mapping and ablation technology.
The following catheters are introduced: quadripolar steerable catheter (Xtrem, Ela Medical, Montrouge, France) positioned in the coronary sinus (CS); and 3.5-mm-tip externally irrigated ablation catheter (Celsius Thermocool, Biosense-Webster, Diamond Bar, CA). The ablation catheter is introduced into the LA through the long sheath, continuously flushed with heparinized saline and maintained within the right atrium whenever it is not required for stability in the LA. Navigation tools, such as LocaLisa (Medtronic, Minneapolis, MN), NavX (Endocardial Solution, Inc., St. Paul, MN), or electroanatomic mapping system (CARTO, Biosense-Webster), can be used adjunctively to fluoroscopy to assist with the procedure and may be helpful to determine the position of the ablation catheter (anterior, posterior) relative to the mapping electrodes. Although these techniques have been demonstrated to significantly reduce the fluoroscopic exposure during linear ablation (8, 9, 10) they also add to the already significant procedural cost.
Following catheter ablation, all antiarrhythmic drugs are discontinued for patients with paroxysmal AF but are continued for 1 to 3 months for patients with permanent AF. Oral anticoagulation is maintained for at least 3 months in patients with paroxysmal AF and 6 months in patients with persistent or permanent AF. After this period, in the absence of arrhythmia, cessation of anticoagulation is considered. A successful outcome is defined as the absence of any atrial arrhythmia after the first month (AF, atrial tachycardia, or flutter) without the use of any antiarrhythmic agents.
Linear ablation within the LA is performed as a means of substrate modification with the aim of joining anatomic structures within the LA in order to create barriers to fibrillatory wave propagation. The impact of linear lesions is reflected by AF cycle length prolongation by a mean of 20 ms and conversion of AF during RF delivery. Although several linear ablation techniques have been performed, presented below are linear lesions that have been recently evaluated at our institution: mitral isthmus ablation (2), ablation at the LA roofline (11), and ablation of the anterior left atrium (12). It is necessary to confirm the completeness of these lines by validated electrophysiologic methods because an incomplete line may not only be ineffective but result in proarrhythmia (13, 14, 15, 16).
Mitral isthmus ablation is performed by creating a linear ablation line joining the lateral mitral annulus to the left inferior PV (2). First, the CS catheter is positioned to bracket the potential linear lesion between its proximal and distal bipoles. The ablation catheter is then introduced through the long sheath to achieve stability; it is bent
with a 90° to 180° curve and ablation is commenced at the ventricular edge of the lateral mitral annulus, where the atrioventricular electrogram shows a 1:1 to 2:1 ratio. The sheath and catheter assembly is then rotated clockwise to extend the lesion to the left inferior PV ostium. Occasionally the proximal end of this ablation line needs to be anchored to a more anterior site and is extended to the posterior root of the left atrial appendage. In general, ablation is commenced at around 3 or 4 o’clock on the mitral annulus and reaches 2 to 3 o’clock at the upper end of the line (Fig. 12.2) and RF energy is delivered for 90 to 120 seconds at each site. Endocardial ablation is performed with a flow rate of 17 to 60 mL/min, target temperature of 45°C, and power of 30 to 37 W. The stability of the catheter needs to be monitored during RF applications using electrograms and intermittent fluoroscopy to avoid inadvertent displacement, which can result in high-energy delivery within the left inferior PV or left atrial appendage. The effect of each RF application is assessed on the local electrogram during pacing from the proximal bipole of the CS catheter (located immediately septal of the line) to maximize conduction delay. Splitting of the local potentials, resulting in an increase in the delay from the pacing artifact, is considered evidence of an effective local lesion. After the initial attempt to create this line, mapping is performed to identify and ablate gaps. Failure to achieve complete block may require a more lateral ablation line near the base of the appendage.
with a 90° to 180° curve and ablation is commenced at the ventricular edge of the lateral mitral annulus, where the atrioventricular electrogram shows a 1:1 to 2:1 ratio. The sheath and catheter assembly is then rotated clockwise to extend the lesion to the left inferior PV ostium. Occasionally the proximal end of this ablation line needs to be anchored to a more anterior site and is extended to the posterior root of the left atrial appendage. In general, ablation is commenced at around 3 or 4 o’clock on the mitral annulus and reaches 2 to 3 o’clock at the upper end of the line (Fig. 12.2) and RF energy is delivered for 90 to 120 seconds at each site. Endocardial ablation is performed with a flow rate of 17 to 60 mL/min, target temperature of 45°C, and power of 30 to 37 W. The stability of the catheter needs to be monitored during RF applications using electrograms and intermittent fluoroscopy to avoid inadvertent displacement, which can result in high-energy delivery within the left inferior PV or left atrial appendage. The effect of each RF application is assessed on the local electrogram during pacing from the proximal bipole of the CS catheter (located immediately septal of the line) to maximize conduction delay. Splitting of the local potentials, resulting in an increase in the delay from the pacing artifact, is considered evidence of an effective local lesion. After the initial attempt to create this line, mapping is performed to identify and ablate gaps. Failure to achieve complete block may require a more lateral ablation line near the base of the appendage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree