Lesions in Small Vessels
Heidar Arjomand
Andrey Espinoza
Sheldon Goldberg
Percutaneous coronary revascularization, mostly by means of stenting, has become the most common major medical intervention (1). One million percutaneous coronary intervention (PCI) procedures are performed annually in the United States. Approximately 30% to 50% of patients undergoing PCI have lesions located in small vessels (reference vessel diameter of <3.0 mm) (1). These patients have been known to have a higher risk of acute complications and recurrent ischemic events after coronary intervention. Moreover, the rate of restenosis is inversely proportional to the reference vessel diameter. Furthermore, the presence of diabetes and complex lesions significantly increases the risk of angiographic restenosis (1,2).
HISTORICAL PERSPECTIVES
Patients with lesions in small coronary vessels have had a historically higher risk of adverse outcomes, with greater rates of periprocedural complications. In a retrospective study of coronary angioplasty procedures, Palacios et al. reported a higher incidence of acute complications, including myocardial infarction (MI) and emergency bypass surgery, in patients undergoing angioplasty of lesions in small vessels (3). In fact, small reference diameter emerged as a strong independent predictor of adverse outcome following balloon angioplasty (BA) (3).
Although the efficacy of coronary stenting (CS) has been proven in patients with a variety of clinical presentations, including acute MI and multivessel coronary artery disease, data on stent placement in patients with lesions in small coronary arteries has been equivocal (1). One potential problem in stenting smaller coronary arteries is the possibility of a higher incidence of stent thrombosis. This concern was initially raised by the data from the French Registry of stent implantation (4). In that study, 2,900 patients who had stent placement were treated with aspirin and ticlopidine. At 30-day follow-up, 51 patients (1.8%) had stent thrombosis, and smaller balloon size was found to be a powerful predictor of this complication. Stent thrombosis occurred in 10% of patients with balloon sizes of 2.5 to 3.0 mm, whereas only 2.3% of patients with balloon sizes of 3.0 to 3.5 mm and 1.0% of patients with balloon sizes of ≥3.5 mm had stent thrombosis (4). However, the concern over a higher incidence of stent thrombosis in patients with smaller coronary vessels has not been observed in other studies (5, 6, 7, 8, 9).
OBSERVATIONAL AND RETROSPECTIVE STUDIES
Initial randomized studies of stent implantation were designed to evaluate the efficacy of stenting, in comparison with BA, in patients with de novo lesions in native coronary vessels of ≥3 mm in diameter or larger (6,7). In a retrospective analysis of the STRESS trial (the STent REStenosis Study), the outcome of stent placement in patients with small coronary vessels (reference diameter of <3.0 mm by quantitative coronary angiography performed in a core angiography laboratory) was evaluated (5). In the STRESS trial, patients with new lesions in native coronary arteries were randomized to receive either Palmaz-Schatz stents or BA. The patients were treated with aspirin, postprocedural heparin, and warfarin (6). In the overall population of 598 patients enrolled in the STRESS I + II trials, 331 patients (56%) were determined to have
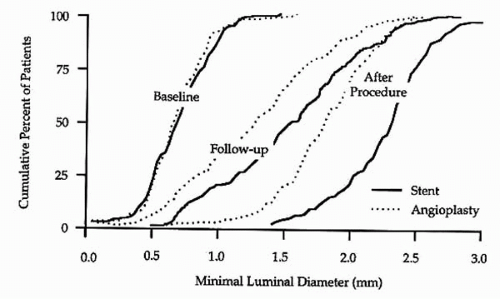
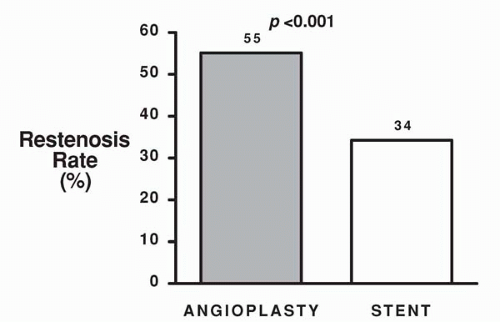
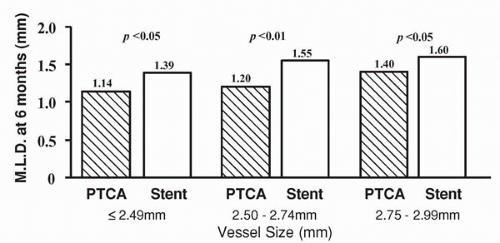
reference vessel diameters of <3.0 mm by quantitative coronary angiographic analysis (5). Of these patients, 163 were randomly assigned to Palmaz-Schatz stent placement and 168 were assigned to BA. A significantly higher procedural success rate was observed for stent placement versus BA (100% versus 92%, p <0.001; Fig. 9.1). This is of particular note when one considers that the stent used in this trial had a bulky delivery system and was not specifically designed for use in smaller coronary vessels. Importantly, the abrupt closure rate (3.6%) was not different for the stent and balloon groups (5). Compared with BA, stent placement resulted in a larger postprocedural lumen diameter (2.26 mm versus 1.80 mm, p <0.001) and a larger lumen at 6-month follow-up (1.54 versus 1.27 mm, p <0.001; Fig. 9.2). Angiographic restenosis was reduced from 55% in patients treated with BA to 34% in patients treated with stent placement (p <0.001; Fig. 9.3). This superior angiographic result was accompanied by improved clinical outcome; 1-year event-free survival was 67% in the balloon group and 78% in the stent group (Fig. 9.4). This difference in the event-free survival was due mainly to a significant reduction in the need for target vessel revascularization. Interestingly, the benefit of stenting relative to BA at 6 months was significant across the range of vessel sizes in terms of follow-up minimal lumen diameter (Fig. 9.5). It has to be noted that this study was a retrospective analysis of data gathered from a prospective randomized trial. Additionally, patients with the tortuous vessels and calcified, diffuse lesions often encountered in clinical practice were excluded from the trial (5).
reference vessel diameters of <3.0 mm by quantitative coronary angiographic analysis (5). Of these patients, 163 were randomly assigned to Palmaz-Schatz stent placement and 168 were assigned to BA. A significantly higher procedural success rate was observed for stent placement versus BA (100% versus 92%, p <0.001; Fig. 9.1). This is of particular note when one considers that the stent used in this trial had a bulky delivery system and was not specifically designed for use in smaller coronary vessels. Importantly, the abrupt closure rate (3.6%) was not different for the stent and balloon groups (5). Compared with BA, stent placement resulted in a larger postprocedural lumen diameter (2.26 mm versus 1.80 mm, p <0.001) and a larger lumen at 6-month follow-up (1.54 versus 1.27 mm, p <0.001; Fig. 9.2). Angiographic restenosis was reduced from 55% in patients treated with BA to 34% in patients treated with stent placement (p <0.001; Fig. 9.3). This superior angiographic result was accompanied by improved clinical outcome; 1-year event-free survival was 67% in the balloon group and 78% in the stent group (Fig. 9.4). This difference in the event-free survival was due mainly to a significant reduction in the need for target vessel revascularization. Interestingly, the benefit of stenting relative to BA at 6 months was significant across the range of vessel sizes in terms of follow-up minimal lumen diameter (Fig. 9.5). It has to be noted that this study was a retrospective analysis of data gathered from a prospective randomized trial. Additionally, patients with the tortuous vessels and calcified, diffuse lesions often encountered in clinical practice were excluded from the trial (5).
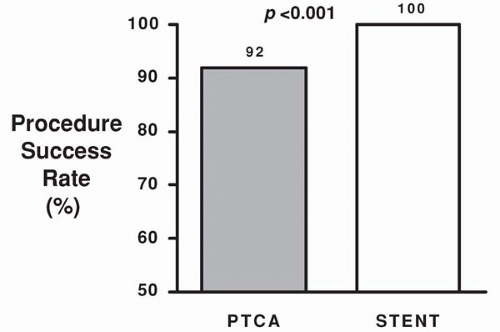 Figure 9.1. Procedural success rate for stent placement versus balloon angioplasty in patients with small vessels in the STRESS I & II trials. |
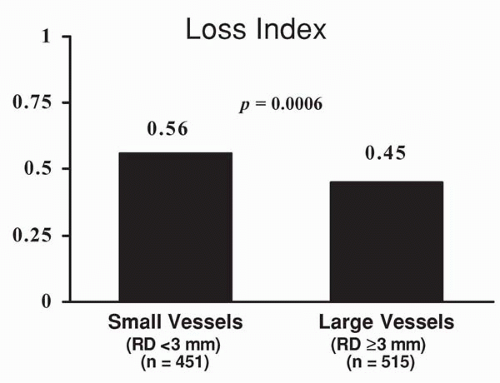
As the application of stent implantation in patients with coronary disease broadened, other studies evaluating the outcome of stenting in patients with smaller coronary vessels emerged (8, 9). In one such study, the outcome of stent placement, using a variety of stents, was compared in 696 patients with larger (>3 mm) coronary arteries versus 602 patients with smaller coronary arteries (reference vessel diameter <3 mm) (8). Angiographic restenosis at 6
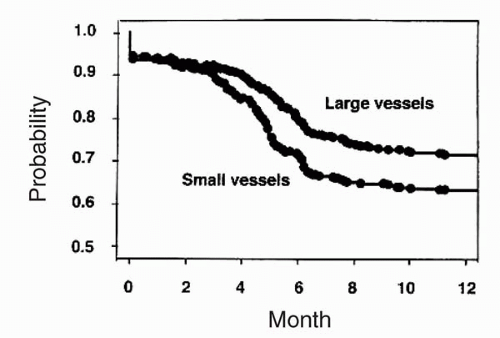
months was significantly higher in patients with smaller coronary vessels (19% versus 33%, p <0.011). As expected, the absolute lumen gain was greater in patients with larger coronary vessels, but absolute lumen loss was similar in the two groups; therefore, the loss index (late loss/acute gain) was less favorable in patients with smaller vessels (Fig. 9.6). Of particular note, the rate of stent thrombosis was similar in patients with larger and smaller coronary vessels (Fig. 9.7). The event-free survival (freedom from death, MI, coronary bypass surgery, or repeat intervention) also was less favorable in patients with smaller reference vessels (Fig. 9.8). In this study, postprocedural stent cross-sectional area was an important predictor of restenosis (8).
months was significantly higher in patients with smaller coronary vessels (19% versus 33%, p <0.011). As expected, the absolute lumen gain was greater in patients with larger coronary vessels, but absolute lumen loss was similar in the two groups; therefore, the loss index (late loss/acute gain) was less favorable in patients with smaller vessels (Fig. 9.6). Of particular note, the rate of stent thrombosis was similar in patients with larger and smaller coronary vessels (Fig. 9.7). The event-free survival (freedom from death, MI, coronary bypass surgery, or repeat intervention) also was less favorable in patients with smaller reference vessels (Fig. 9.8). In this study, postprocedural stent cross-sectional area was an important predictor of restenosis (8).
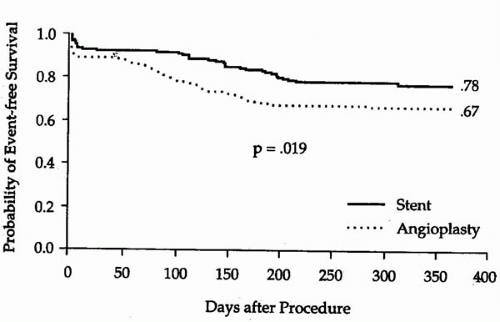 Figure 9.4. Event-free survival in the balloon group (67%) and stent groups (78%) of patients with smaller coronary arteries in the STRESS I & II trials. |
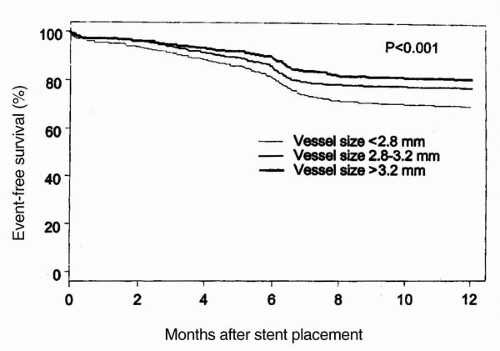
The effect of vessel size on outcome after stent implantation was further assessed in a study of 2,602 patients who had successful stent placement for symptomatic coronary artery disease (9). In that study, Elezi et al. subdivided patients into three equal tertiles of vessel size (<2.8 mm, 2.8 to 3.2 mm, and >3.2 mm). The event-free survival at 1 year was 69.5% in the group with the smallest vessels, 77.5% in the intermediate vessel size, and 81% in the tertile with the largest vessel diameters (p <0.001; Fig. 9.9). The restenosis increased from 20.4% in vessels >3.2 mm to 28.4% in vessels 2.8 to 3.2 mm, and to 38.6% in vessels <2.8 mm (Fig. 9.10). Although patients with the smaller vessels had higher restenosis rates requiring more frequent repeat revascularization, a wide range of restenosis rates was observed within the group of patients with smaller vessels. It was noted that additional important risk factors for the development of restenosis in patients with smaller vessels included patients with a history of diabetes, prior angioplasty, the presence of lesion complexity, and multiple stent placement (9). As shown in Figure 9.11, for patients with vessels <2.8 mm, the restenosis rate was 38.6%; the presence of lesion complexity conferred a higher restenosis rate (41.6%). In patients without complex lesion, however, the restenosis
rate was significantly lower (31.5%). Furthermore, restenosis ranged from 29.6% in patients without diabetes or lesion complexity to 53.5% in patients with both diabetes and complex lesions (9).
rate was significantly lower (31.5%). Furthermore, restenosis ranged from 29.6% in patients without diabetes or lesion complexity to 53.5% in patients with both diabetes and complex lesions (9).
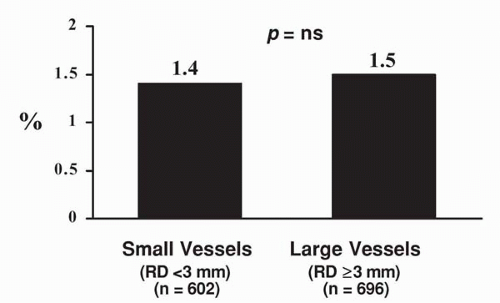 Figure 9.7. Rates of stent thrombosis in patients with small versus large vessels. No difference is observed in the rate of stent thrombosis. |
PROSPECTIVE, RANDOMIZED STUDIES
Data from the aforementioned observational, post hoc analyses were not generated by specifically designed randomized studies comparing stent placement with balloon angioplasty in patients with lesions in small vessels. For this reason, several prospective, randomized trials have evaluated the efficacy of CS, as compared to BA, in small vessels (Table 9.1) (10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23). These trials were either singlecenter or multicenter studies, and enrolled patients with lesions in vessels with reference diameter of <3.0 mm. Several different types of stents were used in these trials (Table 9.2) (23).
In all these trials, crossover from the BA group to the stent group was allowed either as a bailout procedure in the case of abrupt or threatened closure caused by a coronary dissection with compromised antegrade blood flow and/or in the case of a suboptimal result following BA, defined as a residual stenosis of >50% in most studies (23). In a randomized study by Park et al., 120 patients were randomly assigned to either placement of NIR stents or BA. At follow-up angiography, no significant difference in restenosis was observed (35.7% in the CS
group and 30.9% in the BA group, p = NS) (10). In the ISAR-SMART trial, 404 patients were randomly assigned to placement of a Multi-Link stent or BA alone. In this trial, all patients received abciximab. At follow-up, angiographic restenosis was similar in both groups (35.7% versus 37.4%, p = NS) (11). In both these trials, about 20% of patients crossed over from the BA group to the CS group (10, 11). The SISCA trial intended to enroll 200 patients; however, the trial was terminated by the sponsor after inclusion of 145 patients. At 6-month follow-up angiography, a trend was observed in favor of stenting, with lower rate of angiographic restenosis (9.7% versus 18.8% in the BA, p = 0.15) (12). In the BESMART trial, 381 patients were randomized to either placement of a beStent or BA. After 6 months, the restenosis rate was 21% after stenting and 47% after BA (p <0.001). In the BESMART trial, the observed rate of restenosis in the BA group was higher than in the BA group in other trials (13). The Stenting in Small Arteries (SISA) study enrolled 352 patients. The stent used for this trial was also the beStent. After 6 months, no significant difference was observed in the rate of restenosis rate (28% for the CS group and 32.4% for the BA group, p = NS) (14). In the COAST trial, 588 patients were randomized to BA, placement of a bare JoStent (Flex stent), or a heparin-coated JoStent. In this trial, a high (27%) crossover occurred from the BA group to the stent group. At follow-up, the restenosis rate was 27% in the stent groups and 32% in the BA groups (p = NS) (15). In the RAP study, 426 patients were randomly assigned to stent placement with a beStent or BA. At follow-up angiography, the restenosis rate was 27% in the stent group and 37% in the BA group (p <0.05) (16). The CORDIS-MICA trial initially intended to enroll 600 patients; however, the trial was stopped by the sponsor after randomization of 128 patients. The trial had a very high crossover rate of 37% in the BA arm. Based on a very low number of repeat angiographies, mainly driven by recurrent symptoms, restenosis rates were high but not significantly different (61% after stenting and 63% after BA, p = NS) (21). In the LASMAL study, 246 patients were randomized to stenting or BA. A strong trend was observed favoring stent placement in reducing the rate of restenosis (19% in the stent group versus 29% in the BA group) (17). Similarly, a strong trend was observed in favor of stenting (17% versus 25%) for reducing restenosis in the COMPASS trial, which enrolled 100 patients (20). In the SVS trial, 496 patients were randomized to stenting or BA, with no significant difference in the rate of restenosis at follow-up (21% versus 25%, p = NS) (18). In the randomized CHIVAS trial, 141 patients underwent stent placement and 143 patients were treated with BA alone. At follow-up, a significant reduction occurred in the rate of restenosis with stenting (31% versus 43%, p <0.05). Of particular note, in the CHIVAS trial, patients requiring crossover from the BA group to the stent group were excluded from the analysis (19).
group and 30.9% in the BA group, p = NS) (10). In the ISAR-SMART trial, 404 patients were randomly assigned to placement of a Multi-Link stent or BA alone. In this trial, all patients received abciximab. At follow-up, angiographic restenosis was similar in both groups (35.7% versus 37.4%, p = NS) (11). In both these trials, about 20% of patients crossed over from the BA group to the CS group (10, 11). The SISCA trial intended to enroll 200 patients; however, the trial was terminated by the sponsor after inclusion of 145 patients. At 6-month follow-up angiography, a trend was observed in favor of stenting, with lower rate of angiographic restenosis (9.7% versus 18.8% in the BA, p = 0.15) (12). In the BESMART trial, 381 patients were randomized to either placement of a beStent or BA. After 6 months, the restenosis rate was 21% after stenting and 47% after BA (p <0.001). In the BESMART trial, the observed rate of restenosis in the BA group was higher than in the BA group in other trials (13). The Stenting in Small Arteries (SISA) study enrolled 352 patients. The stent used for this trial was also the beStent. After 6 months, no significant difference was observed in the rate of restenosis rate (28% for the CS group and 32.4% for the BA group, p = NS) (14). In the COAST trial, 588 patients were randomized to BA, placement of a bare JoStent (Flex stent), or a heparin-coated JoStent. In this trial, a high (27%) crossover occurred from the BA group to the stent group. At follow-up, the restenosis rate was 27% in the stent groups and 32% in the BA groups (p = NS) (15). In the RAP study, 426 patients were randomly assigned to stent placement with a beStent or BA. At follow-up angiography, the restenosis rate was 27% in the stent group and 37% in the BA group (p <0.05) (16). The CORDIS-MICA trial initially intended to enroll 600 patients; however, the trial was stopped by the sponsor after randomization of 128 patients. The trial had a very high crossover rate of 37% in the BA arm. Based on a very low number of repeat angiographies, mainly driven by recurrent symptoms, restenosis rates were high but not significantly different (61% after stenting and 63% after BA, p = NS) (21). In the LASMAL study, 246 patients were randomized to stenting or BA. A strong trend was observed favoring stent placement in reducing the rate of restenosis (19% in the stent group versus 29% in the BA group) (17). Similarly, a strong trend was observed in favor of stenting (17% versus 25%) for reducing restenosis in the COMPASS trial, which enrolled 100 patients (20). In the SVS trial, 496 patients were randomized to stenting or BA, with no significant difference in the rate of restenosis at follow-up (21% versus 25%, p = NS) (18). In the randomized CHIVAS trial, 141 patients underwent stent placement and 143 patients were treated with BA alone. At follow-up, a significant reduction occurred in the rate of restenosis with stenting (31% versus 43%, p <0.05). Of particular note, in the CHIVAS trial, patients requiring crossover from the BA group to the stent group were excluded from the analysis (19).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree