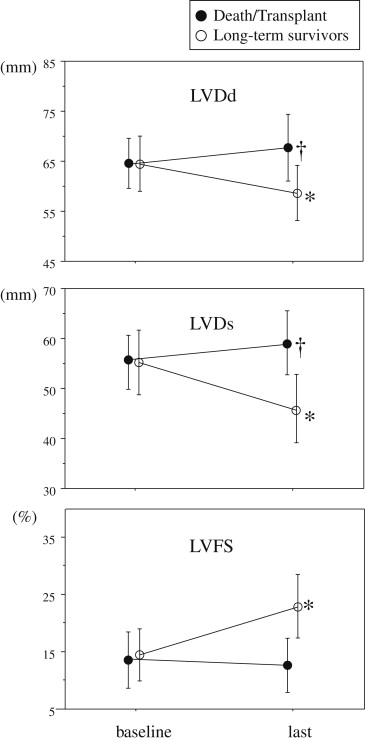
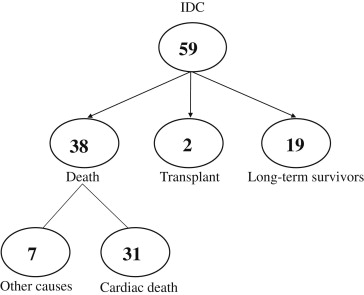
Little is known about left ventricular (LV) reverse remodeling (LVRR) in long-term survivors with idiopathic dilated cardiomyopathy. We studied 59 patients with idiopathic dilated cardiomyopathy who had a potential clinical and echocardiographic follow-up period of >12 years. LVRR was defined as LV end-diastolic dimension ≤55 mm and fractional shortening ≥25% on the last echocardiogram. Of the 59 patients, 38 died (heart failure in 20, sudden death in 11, and other causes in 7), 2 underwent transplantation, and 19 survived. In the survivors, the LV size had significantly decreased and LV fractional shortening had significantly increased on the last echocardiogram. LVRR occurred in 37% of the survivors. The remaining 63% of the survivors still had LV dysfunction, but the LV end-systolic dimension had decreased significantly. In patients who died or underwent transplantation, the LV size significantly increased. No patient who died or underwent transplantation had LVRR. In conclusion, >60% of the long-term (>12 years) survivors with idiopathic dilated cardiomyopathy still had LV systolic dysfunction, but the LV end-systolic dimension had decreased significantly. In contrast, patients who died or underwent transplantation had significant LV enlargement. These results suggest that LVRR, even if it is not marked, is associated with a favorable prognosis.
Idiopathic dilated cardiomyopathy (IDC) is characterized by left ventricular (LV) dilation with systolic dysfunction. The prognosis of patients with IDC has markedly improved. We previously reported that >50% of the patients with IDC survived for 10 years. Reverse remodeling, which is a decrease in LV size with improvement in systolic function, has an important role in the prognosis of IDC. Patients with IDC who will have a favorable prognosis can be identified by LV reverse remodeling (LVRR) at short- or mid-term follow-up. Nevertheless, few data are available on LVRR in long-term survivors with IDC. The aim of the present study was to investigate the changes in LV size and systolic function in patients with IDC who had survived for >12 years.
Methods
We retrospectively studied 59 patients with IDC who had been admitted to the Kochi Medical School Hospital from 1983 to 1999. On admission, an exhaustive clinical evaluation, including medical history, physical examination, 12-lead electrocardiography, ambulatory 24-hour electrocardiography, laboratory studies, echocardiography, and cardiac catheterization, was performed in each patient to identify the cause of cardiomyopathy as precisely as possible. The diagnostic criterion was a dilated LV end-diastolic dimension (LVDd) >55 mm with fractional shortening (FS) <25%. Patients with acute myocarditis, infiltrative myocardial disease, connective tissue disease, endocrine dysfunction, neuromuscular disease, general systemic disease, significant coronary artery stenosis (defined as diameter narrowing >50% in any of the major coronary arteries or their branches), valvular disease, sensitivity/toxic reactions, or a history of excessive alcohol intake were excluded. The LVDd, LV end-systolic dimension (LVDs), thickness of the interventricular septum and LV posterior wall, and left atrial dimension were measured on the M-mode echocardiogram, as recommended by the American Society of Echocardiography. LVFS was calculated as [(LVDd − LVDs)/LVDd] × 100. Nonsustained ventricular tachycardia was defined as ≥3 consecutive premature complexes at a mean rate >120 beats/min. The estimated glomerular filtration rate was calculated. The study patients had a potential clinical and echocardiographic follow-up period of >12 years and had undergone echocardiography at baseline and within 1 year of their last visit, death, or transplantation. The Ethics Committee on Medical Research of the Kochi Medical School approved the present study. All patients gave informed consent. Follow-up data were obtained by regular visits and chart reviews and telephone interviews with the patients or their relatives. Echocardiography was performed in routine clinical practice. LVRR was defined as described previously : LVDd ≤55 mm, with LVFS ≥25% on the last echocardiogram. The end points were death or heart transplantation.
Categorical variables are presented as the total number and percentages of patients and continuous variables as the mean ± SD. Fisher’s exact test was used to analyze the categorical variables. The differences in continuous variables were analyzed using the unpaired Student’s t test or the Mann-Whitney U test, as appropriate. Changes in the LVDd, LVDs, and LVFS were evaluated using the Wilcoxon signed-rank test. p Values <0.05 were considered statistically significant.
Results
During a follow-up period of 9.8 ± 6.9 years (range 5 months to 26 years), 38 patients died (heart failure in 20, sudden death in 11, and other causes in 7), 2 underwent heart transplantation, and 19 survived for >12 years ( Figure 1 ). A cardiac resynchronization therapy device with a defibrillator was implanted in 1 patient who died of heart failure and in 1 survivor. The 7 patients who died of noncardiac causes were excluded from the present analysis.
We divided the patients into 2 groups: those who had died from cardiac causes or had undergone heart transplantation (death or transplantation) and those who had survived for >12 years (long-term survivors). No significant differences in the patient characteristics were found between the 2 groups, except for the follow-up period and the frequency of use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers ( Table 1 ). We most frequently used enalapril as the angiotensin-converting enzyme inhibitor and losartan as the angiotensin II receptor blocker. The daily dose was 5.0 ± 1.1 mg (range 2.5 to 10) for enalapril and 43.0 ± 12.2 mg (range 25 to 50) for losartan. No difference in the frequency of use of β blockers was found. The daily dose was 10.0 ± 5.2 mg (range 2.5 to 20) for carvedilol and 65.2 ± 22.8 mg (range 40 to 80) for metoprolol. The angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and β blockers were continued during follow-up, but some changes, including other concomitant drugs such as diuretics, occurred, when clinically indicated. No significant differences in echocardiographic variables were found ( Table 2 ).
| Variable | Death or Transplantation (n = 33) | Long-term Survivors (n = 19) | p Value |
|---|---|---|---|
| Age (yrs) | 58 ± 11 | 58 ± 7 | 0.884 |
| Men | 24 (73%) | 16 (84%) | 0.343 |
| New York Heart Association class | 0.067 | ||
| I–II | 19 | 16 | |
| III–IV | 14 | 3 | |
| Atrial fibrillation | 2 (6%) | 1 (5%) | 0.905 |
| Nonsustained ventricular tachycardia | 14 (33%) | 4 (21%) | 0.118 |
| Serum creatinine (mg/dl) | 0.90 ± 0.29 | 0.85 ± 0.03 | 0.530 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 72.6 ± 12.7 | 72.4 ± 12.7 | 0.938 |
| Serum hemoglobin (g/dl) | 14.0 ± 3.1 | 14.6 ± 2.6 | 0.255 |
| Follow-up period (years) | 6.0 ± 5.1 | 16.4 ± 4.3 | <0.001 |
| Medical treatment | |||
| β Blockers | 12 (36%) | 10 (53%) | 0.252 |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 14/6 (61%) | 12/7 (100%) | 0.001 |
| Loop diuretics | 27 (82%) | 17 (89%) | 0.252 |
| Spironolactone | 20 (61%) | 6 (32%) | 0.072 |
| Digitalis | 27 (82%) | 14 (74%) | 0.489 |
| Amiodarone | 1 (3%) | 1 (5%) | 0.921 |
| Variable | Death or Transplantation | Long-term Survivors | p Value |
|---|---|---|---|
| Left ventricular end-diastolic dimension (mm) | 65 ± 5 | 65 ± 6 | 0.932 |
| Left ventricular end-systolic dimension (mm) | 56 ± 6 | 55 ± 7 | 0.746 |
| Left ventricular fractional shortening (%) | 14 ± 5 | 15 ± 5 | 0.742 |
| Interventricular septal thickness (mm) | 10 ± 1 | 10 ± 1 | 0.382 |
| Left ventricular posterior wall thickness (mm) | 10 ± 1 | 11 ± 2 | 0.273 |
| Left atrial dimension (mm) | 44 ± 7 | 40 ± 6 | 0.633 |
| Left ventricular end-diastolic volume index (ml/m 2 ) | 168 ± 49 | 159 ± 61 | 0.282 |
| Left ventricular end-systolic volume index (ml/m 2 ) | 118 ± 42 | 108 ± 57 | 0.214 |
| Left ventricular ejection fraction (%) | 30 ± 9 | 33 ± 9 | 0.305 |
| Left ventricular end-diastolic pressure (mm Hg) | 18 ± 9 | 13 ± 6 | 0.041 |
| Pulmonary capillary wedge pressure (mm Hg) | 13 ± 8 | 10 ± 5 | 0.309 |
| Systolic pulmonary artery pressure (mm Hg) | 35 ± 11 | 27 ± 7 | 0.163 |
| Mean pulmonary artery pressure (mm Hg) | 22 ± 10 | 19 ± 7 | 0.235 |
| Right ventricular end-diastolic pressure (mm Hg) | 8 ± 5 | 8 ± 2 | 0.791 |
| Mean right atrial pressure (mm Hg) | 7 ± 5 | 6 ± 2 | 0.648 |
| Systolic aortic pressure (mm Hg) | 113 ± 18 | 122 ± 19 | 0.129 |
| Mean aortic pressure (mm Hg) | 88 ± 13 | 92 ± 12 | 0.266 |
| Cardiac index (ml/min/m 2 ) | 2.2 ± 0.6 | 2.5 ± 0.5 | 0.053 |
In the long-term survivors, the LV size had significantly decreased and the LVFS had significantly increased on the last echocardiogram ( Figure 2 ). Of the 19 survivors, 7 (37%) showed LVRR (LVDd decreased from 66 ± 6 to 50 ± 2 mm, p = 0.004; LVDs decreased from 57 ± 7 to 33 ± 4 mm, p <0.001; and LVFS increased from 14 ± 3% to 33 ± 5%, p <0.001). The remaining 12 survivors (63%) still had LV dysfunction, but a significant decrease in the LVDs was noted (LVDd 64 ± 5 to 62 ± 5 mm, p = 0.075; LVDs 55 ± 6 to 51 ± 6 mm, p = 0.020; LVFS 14 ± 5% to 18 ± 5%, p = 0.062). Of the 12 survivors without LVRR, 11 were in New York Heart Association functional class II and 1 in class III. In the death or transplantation group, the left ventricle showed significant enlargement on the last echocardiogram ( Figure 2 ). No patient who died or underwent transplantation showed LVRR, in contrast to the long-term survivors (0% vs 37%, p <0.001).