, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
Aortic valve stenosis refers to a narrowing of the lumen of the aortic valve. It is the most common cardiac valvular condition requiring valve replacement. Congenital stenosis is the most common cause of aortic valve stenosis. It is usually associated with anomalous valves, most commonly the bicuspid aortic valve. The congenitally stenotic aortic valve also may be unicuspid (acommissural or unicommissural) or, rarely, congenitally quadricuspid/quadricommissural. A dysplastic tricuspid aortic valve can also develop stenosis (Fig. 13.1).
13.1 Aortic Valve Stenosis
Aortic valve stenosis refers to a narrowing of the lumen of the aortic valve. It is the most common cardiac valvular condition requiring valve replacement. Congenital stenosis is the most common cause of aortic valve stenosis. It is usually associated with anomalous valves, most commonly the bicuspid aortic valve. The congenitally stenotic aortic valve also may be unicuspid (acommissural or unicommissural) or, rarely, congenitally quadricuspid/quadricommissural. A dysplastic tricuspid aortic valve can also develop stenosis (Fig. 13.1).
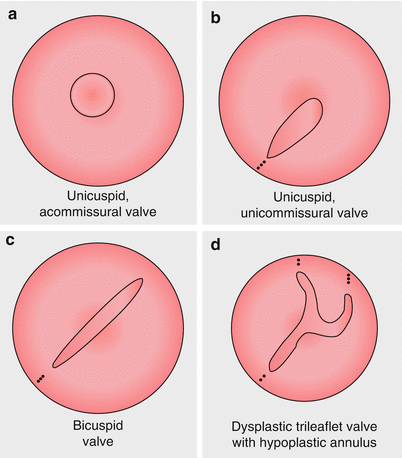

Fig. 13.1
Morphologic variants of congenital aortic stenosis: Panel (a) unicuspid, acommissural valve. Panel (b) unicuspid, unicommissural valve. Panel (c) bicuspid valve. Panel (d) dysplastic, trileaflet valve with hypoplastic annulus
13.1.1 Bicuspid Aortic Valve
Bicuspid aortic valve disease is the most common congenital heart defect, occurring in 0.5–2 % of the general population, with a 3:1 male predominance [1]. Morphologically, there is a single commissure at birth with two unequally sized leaflets. The incompletely separated leaflet has a central ridge (raphe). Typically, the bicuspid valve results from fusion of the right and left cusps. The noncoronary leaflet is most commonly normal. Rarely, the leaflets are symmetrical or there is no raphe (“pure” bicuspid valve). The most commonly associated anomalies are coarctation of the aorta and interrupted aortic arch, and conversely 50–75 % of patient with coarctation have a bicuspid valve [1, 2]. Other associated conditions include hypoplastic left heart syndrome, Shone syndrome, Williams syndrome, supravalvular stenosis, Turner syndrome, ventricular and atrial septal defects, patent ductus arteriosus, and single coronary artery or reversal of coronary dominance [2].
In the adult population, it is important to distinguish between a bicuspid valve and a tricuspid aortic valve prior to transcatheter aortic valve implantation as the bicuspid valve currently is considered a contraindication to this procedure in the U.S. Frequently, distinguishing a bicuspid aortic valve from a tricuspid, normal valve is difficult using echo due to heavy calcification of the aortic valve. CT can be helpful in this setting. CT is also useful to evaluate aortic valve size and valve opening area.
To diagnose a bicuspid valve, the valve must be visualized in systole, since in diastole the raphe may mimic a tricuspid valve. CT has been demonstrated to be highly sensitive and specific (94 and 100 %) for accurate differentiation between bicuspid and tricuspid valves [3]. Retrospective ECG-gated data acquisition through the entire cardiac cycle is necessary for this purpose.
The classic CT finding of a bicuspid aortic valve is the presence of only two cusps [4]. In the axial plane, the orifice has a characteristic “fish-mouthed” appearance. In the long-axis view, the eccentric closure line and doming of the leaflets are observed. The two cusps are commonly asymmetric in size with one cusp being larger than the other. The cusps may be thickened and calcified, resulting in reduction of the aortic valve area (Figs. 13.2 and 13.3).
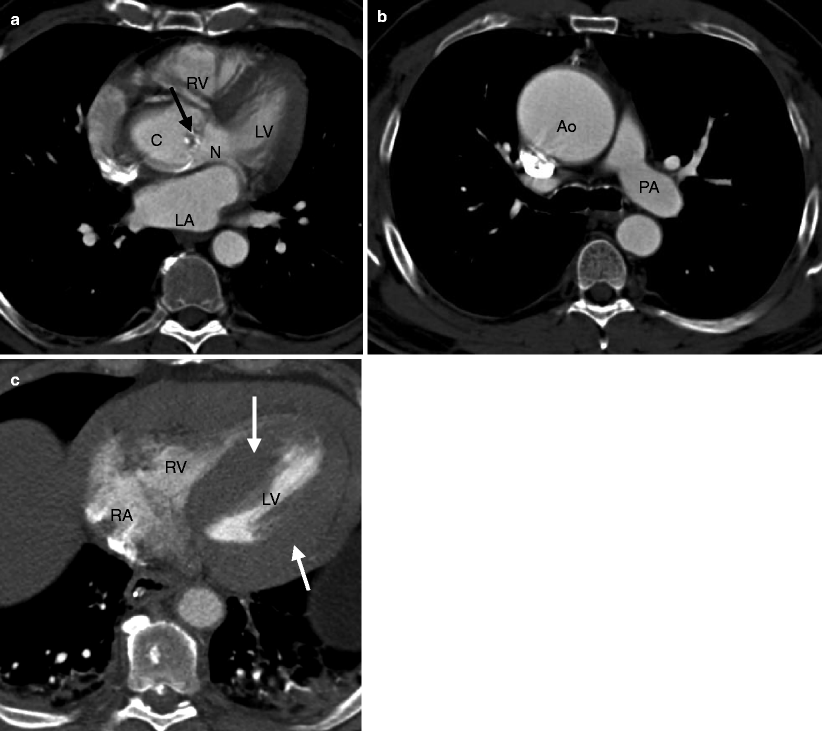
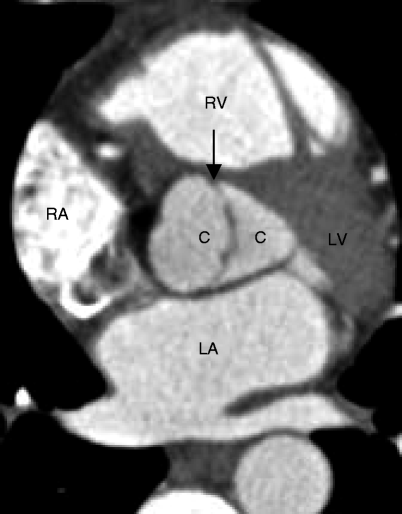

Fig. 13.2
Bicuspid aortic valve in systole. Panel (a) is a short-axis computed tomogram in systole showing two asymmetric cusps and calcified raphe (arrow). The larger cusp (C) represents the fused right and left cusps. N noncoronary cusp. Panel (b) is an axial view reconstructed more superiorly and shows a dilated ascending aorta (Ao). Panel (c) is an axial cut at the level of the left ventricle. Note the profound left ventricular hypertrophy (white arrows). RV right ventricle, LV left ventricle, LA left atrium, RA right atrium, PA pulmonary artery

Fig. 13.3
This short-axis scan demonstrates nearly symmetric bileaflet aortic valve cusps (C) and a thickened commissure (black arrow). Two symmetric-size cusps are less common than two asymmetric-size cusps: RA right atrium, RV right ventricle, LA left atrium, LV left ventricle
The presence of valvular calcifications can cause artifacts, but with CT these usually do not hinder the planimetric measurement of valve area. Studies have shown that planimetric CT measurements of the aortic valve area correlate significantly with transthoracic echocardiographic and transesophageal echocardiographic measurements [5, 6]. Aortic valve calcification can be quantified and is associated with the severity of aortic stenosis [7]. Cine images may show decreased excursion of the valve cusps.
Associated findings related to a bicuspid aortic valve include left ventricular hypertrophy and dilatation of the aortic root as well as ascending aorta and anomalies such as aortic coarctation and interrupted aortic arch. See Table 13.1.
Table 13.1
CT assessment of a bicuspid aortic valve
Retrospective ECG-gated data acquisition through entire cardiac cycle |
Two valvular cusps with “fish-mouthed” orifice appearance, axial images |
Doming of the valve, long-axis reconstructions |
Dilated ascending aorta |
Planimetry of aortic valve area with cine images to show valve excursion |
Left ventricle hypertrophy, systolic function |
Coronary artery anatomy |
Associated lesions: commonly aortic coarctation and interrupted aortic arch |
The natural history of bicuspid aortic valve consists of gradual calcification and degeneration of the valve leaflets resulting in aortic stenosis most commonly and sometimes aortic regurgitation. These findings occur most often in the fifth and sixth decades of life. Aortopathy consisting of dilatation of the aortic root and or ascending aorta can also develop In addition to valvular degeneration, aortic regurgitation may also occur secondary to ascending aorta and aortic root dilatation, which do commonly occur. In the Olmsted County study, up to 39 % of the population with bicuspid aortic valve demonstrated an ascending aorta diameter over 40 mm [8].
Surgical intervention is necessary in patients with bicuspid aortic valve and symptomatic aortic stenosis or aortic regurgitation. Approximately 3–6 % of patients will require surgical intervention for severe symptomatic regurgitation [8]. Surgical intervention usually consists of aortic valve replacement. For those aortic stenosis patients who are not surgical candidates and who do not have significant aortic regurgitation, balloon valvuloplasty may be performed. Balloon valvuloplasty is generally not indicated in the setting of heavy valvular calcification.
Surgical correction of symptomatic pediatric and young adolescent patients with a bicuspid aortic valve is sometimes performed using the Ross procedure (aortic valve replacement with an autologous pulmonary homograft and pulmonary valve replacement with a cadaveric allograft).
Surgery to correct the aortic root without valve replacement is performed in approximately 25 % of patients with bicuspid aortic valve [9]. In patients with a stenotic bicuspid aortic valve and ascending aortic or root aneurysm (maximum diameter > 4.5 cm), concomitant aortic root replacement may be performed at the time of valve replacement. Because aortopathy is an associated characteristic of bicuspid aortic valves, the threshold for aortic aneurysm repair is reduced and smaller diameter aortas are replaced.
13.1.2 Dysplastic Tricuspid Aortic Valve
A dysplastic tricuspid aortic valve may develop concomitant thickening, stenosis, and calcification. The cusps of the valve may themselves be thickened, rolled, and redundant. Microscopic studies reveal that they consist of immature loose connective tissue. The valve orifice is obstructed by the dysplastic cusps. Dysplastic changes rather than commissural fusion are responsible for the functional aortic stenosis. These valves are generally not amenable to valvulotomy because obstruction is due to abnormal valve tissue.
13.1.3 Unicuspid Aortic Valve
Unicuspid aortic valve is an uncommon condition with an incidence of 0.02 % in the general population [10]. It is more common in men than women [10]. These valves form due to fusion of the cusps. Unicuspid valves are usually unicommissural with a posterior commissural attachment. Rarely, they are acommissural. This anomaly is commonly associated with aortic stenosis which is often asymptomatic. In asymptomatic patients, the diagnosis is most commonly made either at autopsy or by examination of surgically excised valves (60 % of cases) [11].
Unicuspid aortic valves share many of the features of bicuspid aortic valves (valvular dysfunction, aortopathy), but complications occur earlier in life and progress at a faster pace. This anomaly should be suspected in patients presenting at a young age with clinically significant aortic stenosis. CT can confirm the valve morphology and demonstrate the absence of a commissure [10] (Fig. 13.4).
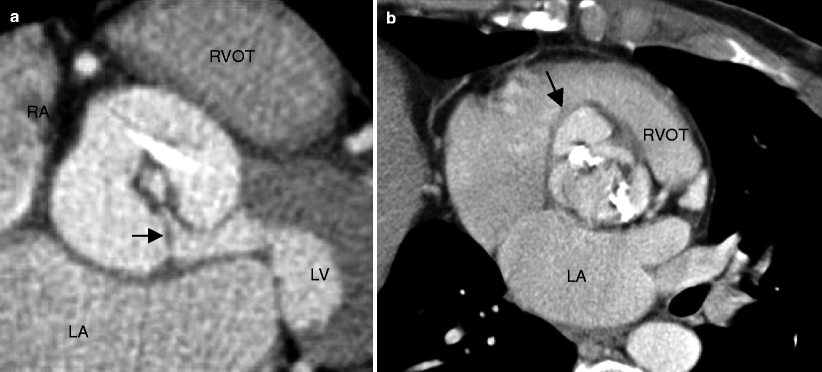

Fig. 13.4
Panel (a) is an example of a unicuspid aortic valve resulting in symptomatic aortic stenosis in a middle-aged adult. This computed tomography angiogram shows a unicommissural (arrow) unicuspid valve. Note the circular, small valve opening in systole in the center of the image (Panel a was generously provided by Dr Jeffry M Schussler, Baylor University Medical Center). Panel (b) is an axial, oblique multiplanar reconstruction illustrating the fused leaflets typical for a unicuspid aortic valve. Panel (b) points to a coexisting sinus of Valsalva aneurysm (black arrow). RVOT right ventricular outflow tract, RA right atrium, LA left atrium, LV left ventricle (Panel (b) is reproduced from Dandes [35]. With kind permission of Springer-Verlag, Berlin Heidelberg)
13.1.4 Quadricuspid Aortic Valve
Quadricuspid aortic valve is a rare congenital defect. It has no gender propensity. Typically, there are three equal-sized cusps with a fourth, smaller cusp. Aortic regurgitation commonly results (75 % of cases) and is caused by malcoaptation of the four valvular leaflets [12]. About 16 % of patients exhibit normal valve function [12]. Aortic stenosis, the most frequent complication of bicuspid valve, is less frequently seen with quadricuspid aortic valve. Quadricuspid aortic valves have been associated with patent ductus arteriosus, Ehlers–Danlos syndrome, hypertrophic obstructive cardiomyopathy, and subaortic stenosis. CT can depict the morphology of the valve and can also provide information about the presence of stenosis and regurgitation when viewed in cine mode [13]. See Fig. 13.5.
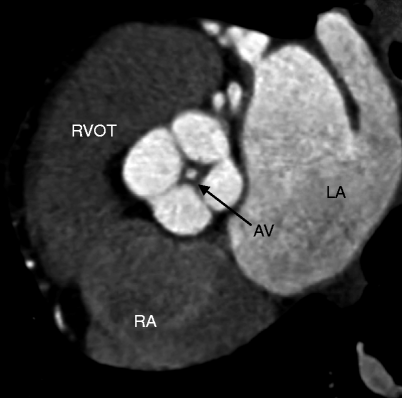

Fig. 13.5
Quadricuspid aortic valve. An oblique axial slice in diastole shows four valve leaflets (two equal-sized large and two equal-sized smaller valve leaflets) with mild thickening and incomplete coaptation and central aortic insufficiency indicated by the circular contrast density centered in the valve orifice (labeled AV) during diastole. LA left atrium, RA right atrium, RVOT right ventricular outflow tract
13.1.5 Subaortic Stenosis in Adults
Subaortic stenosis is considered an acquired (rather than congenital) cardiac defect of postnatal development since it does not appear during embryologic development of the heart and occurs very infrequently in the neonatal period. The prevalence is approximately 6.5 % for all adults with congenital heart disease and 44 % have other associated congenital heart diseases [14]. It usually presents after the first year of life and causes severe left ventricular outflow tract obstruction (LVOT) and the development of heart failure during the first year of life.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


