, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
Left ventricular noncompaction (LVNC), also called spongiform cardiomyopathy, is a rare congenital cardiomyopathy that results from interrupted cardiac embryogenesis. Pathologically, it is characterized by prominent trabeculations and deep intertrabecular recesses, creating a “spongy” myocardium. The cause is thought to be an arrest in the gradual compaction of the loosely interwoven meshwork of myocardial fibers during endomyocardial morphogenesis at 5–8 weeks of fetal life. The condition predominately affects the left ventricle (LV).
Left ventricular noncompaction (LVNC), also called spongiform cardiomyopathy, is a rare congenital cardiomyopathy that results from interrupted cardiac embryogenesis. Pathologically, it is characterized by prominent trabeculations and deep intertrabecular recesses, creating a “spongy” myocardium. The cause is thought to be an arrest in the gradual compaction of the loosely interwoven meshwork of myocardial fibers during endomyocardial morphogenesis at 5–8 weeks of fetal life. The condition predominately affects the left ventricle (LV).
Engberding and Bender first described LVNC as an isolated condition in 1984 [1]. Subsequently LVNC has been associated with other congenital anomalies such as obstruction of the right or left ventricular outflow tracts, complex cyanotic congenital heart disease, coronary artery anomalies, hypertrophic and restrictive cardiomyopathies, and recently with ARVD.
Originally reported to be present in only 0.05 % of adults [2], it is more frequently recognized primarily as result of high-resolution images of the left ventricular apex afforded by magnetic resonance imaging (MR) and computed tomography (CT) as compared to echocardiography. More recently, LVNC was reported in 2 % of patients undergoing MDCT for assessment of coronary artery disease [3]. LVNC may be associated with development of systolic and diastolic LV dysfunction, arrhythmias, congestive heart failure, and thromboembolic events. The age of onset and degree of clinical symptoms depend on the extent of the noncompacted cardiac segments [4].
Traditionally, echocardiography has been used to establish the diagnosis of myocardial noncompaction, although CT and MRI provide better visualization of the trabeculations. CT findings of LVNC are prominent trabeculations and deep recesses in the myocardium usually affecting the apical and basal surfaces of the left ventricle. The RV apex also may be involved [5, 6]. Based on MRI criteria, a noncompacted to compacted myocardium ratio (NC/C ratio) of 2:1 at end systole (or 2.3:1 in end diastole in the long axis) supports the diagnosis of LVNC. A recent, small CT study suggests that a ratio of noncompacted to normal myocardium of 2.2:1 in more than one myocardial segment suggests the diagnosis of LVNC [7]. Left ventricular systolic dysfunction and restrictive filling may be seen on cine imaging. CT examples of LVNC are presented in Figs. 9.1 and 9.2.
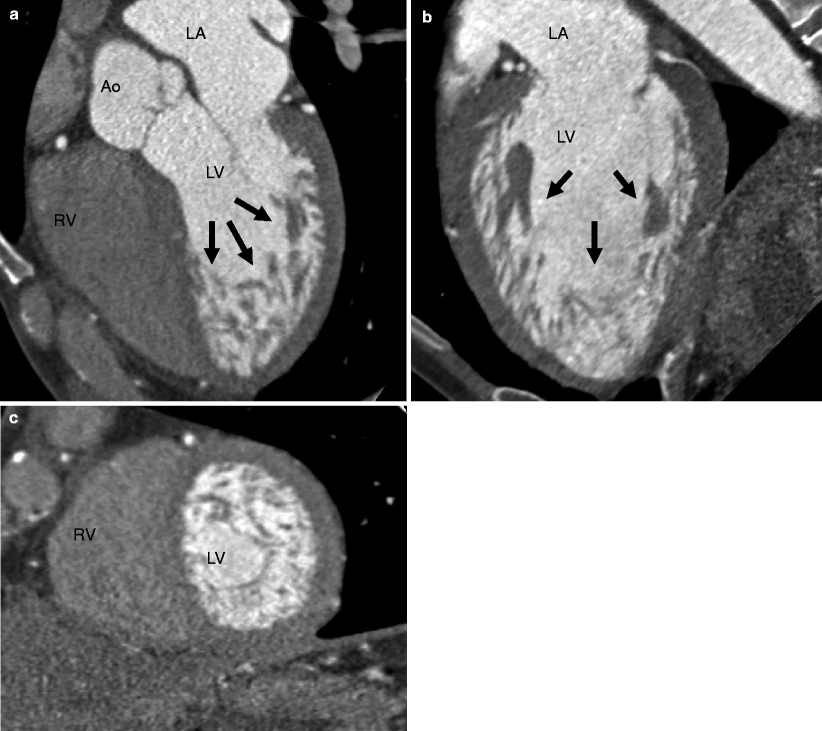

Fig. 9.1




Left ventricular noncompaction (LVNC) in an asymptomatic marathon runner. Panel (a) is a five-chamber view, panel (b) is a two-chamber orientation, and panel (c) is a short-axis cut. Note the dilated left ventricle and the fine trabeculations with deep intertrabecular recesses (arrows) in the myocardium on the apical regions of the left ventricle. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle, Ao aorta
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


