Fig. 13.1
Routine ECG. Notice the normal sinus rhythm without abnormalities in conduction or repolarization
Chest X-Ray
A chest x-ray was performed too (Fig. 13.2): cardiac shadow was slightly enlarged with an increase of cardiac transverse diameter with left ventricular preponderance. A left-sided pleural effusion was seen obliterating the costophrenic recess and was associated to a bilateral hilar enlargement with widespread bronchovascular signs.
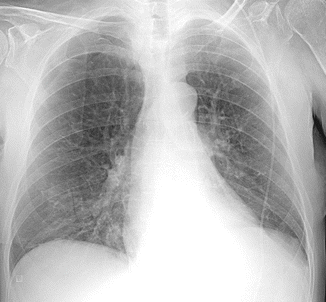

Fig. 13.2
Chest x-ray performed for the worsening dyspnea. The bronchovascular marking is evident
Routine Laboratory Tests
Complete blood count: normal
Cholesterol (total, HDL, LDL) and TG : normal
Hepatic function (GOT, GPT, γ-GT, ALP, total bilirubin, direct and indirect): normal
Thyroid function (TSH, FT3, FT4): normal
Renal function (creatinine, BUN): normal
Electrolytes (Na + , K + , Ca ++ , Mg ++ , Cl − ): normal
Fasting blood glucose: 154 m/dl (8.56 mmol/L)
HbA1c: 7.1 % (54 mmol/mol)
TnI-hs and CK-MB: normal
BNP: 673 pg/ml
What Are the Possible Causes of Heart Failure?
Myocardial disease
Coronary artery disease
Hypertension
Cardiomyopathy
Familial (hypertrophic, dilated, ARVD, restrictive, left ventricular non-compaction)
Myocarditis (infective, immune-mediated, chemotherapy, cocaine, alcohol, heavy metals)
Endocrine/nutritional (pheochromocytoma, vitamin deficiency, hypophosphatemia, hypocalcemia)
Pregnancy
Infiltration (amyloidosis, malignancy)
Valvular heart disease
Mitral
Aortic
Tricuspid
Pulmonary
Pericardial disease
Constrictive pericarditis
Pericardial effusion
Congenital heart disease
Arrhythmias
Tachyarrhythmias (atrial, ventricular)
Bradyarrhythmias (sinus node dysfunction, atrioventricular block)
High output states
Anemia
Sepsis
Thyrotoxicosis
Paget’s disease
Arteriovenous fistula
Volume overload
Renal failure
Iatrogenic (postoperative fluid infusion)
The diagnosis of heart failure is now very likely according to symptoms, physical exam, chest radiography, and laboratory tests.
Echocardiography
Echocardiography was also performed to evaluate function and morphology of cardiac valves and the left ventricle (Fig. 13.3a, b).
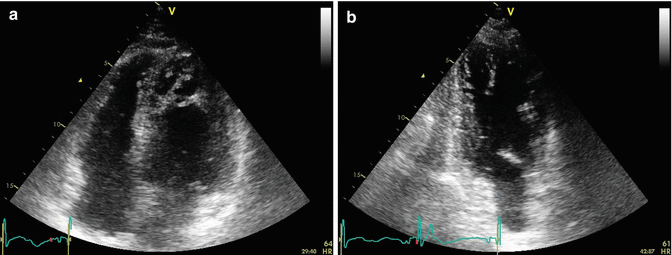

Fig. 13.3
Echocardiogram: (a) apical four-chamber view. The marked trabeculae of the apex can be noticed. (b) Apical two-chamber view. Apex trabeculae are visible also in this view
Aortic valve was normal and trileaflet. Normal dimensions of the left atrium (LA diameter M-mode, 3.9 cm; area 4c, 13 cm2). The mitral valve, tricuspid valve, and pulmonic valve had normal function and morphology; the right atrium was also normal (area 4c = 13 cm2). The right ventricle was normal in size with normal function (TAPSE 22 mm). There was a mild tricuspid regurgitation centrally directed. There was no evidence of atrial septal defects. The left ventricle was slightly dilated (indexed left end-diastolic volume 80 ml/m2) with severe reduction of systolic function and areas of prominent trabeculae in the apex and the side wall. Ejection fraction measured with Simpson’s biplane method was 30 %. The aorta was normal (aortic root dimension, 2.7 cm; ascending aorta, 3.1 cm; aortic arch, 3.1 cm; thoracic aorta, 2.8 cm; abdominal aorta, 1.8 cm). IVC was slightly dilated with an inspiratory collapse <50 % (RAP =10 mmHg and PAPS = 35 mmHg).
Conclusions: There is severe reduction of left ventricular function. Left ventricle walls are homogeneously hypokinetic. Morphologic alterations of the apex and side wall of the left ventricle compatible with non-compaction of the myocardium.
Because of that morphologic pattern of the left ventricle, a cardiac RMN was requested (Fig. 13.4a–f). “[…] Prominent endocardic apical-subapical trabeculae, extended to the side wall and the middle of LV, compared to a compact epicardial layer (Noncompaction/compaction ratio 2.3). Those findings can be related to areas of myocardial non-compaction.”
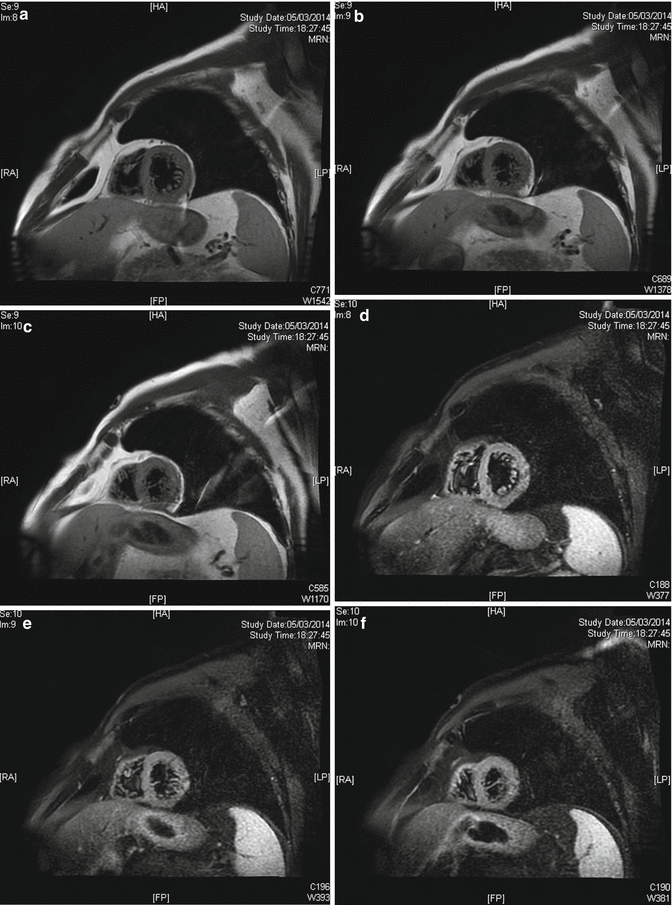

Fig. 13.4
(a–f) Cardiac RMN, short-axis view of the left ventricle in different levels (T1-weighted black blood imaging). In these images, trabecula’s extension to the side wall is visible
The patient was diagnosed with left ventricular non-compaction cardiomyopathy.
The patient was monitored, and an intravenous diuretic therapy was introduced with clinical benefit. Symptoms resolved and dyspnea and fatigue disappeared.
Now the Heart Failure Is Explained. But What About the Syncope? What Are the Possible Causes?
Neurally mediated syncope (vasovagal, situational, carotid sinus syncope)
Orthostatic hypotension (primary autonomic failure as Parkinson’s disease or secondary autonomic failure as diabetes or amyloidosis, drug-induced, volume depletion)
Cardiac syncope (bradyarrhythmias, tachyarrhythmias as supraventricular or ventricular tachycardia, drug-induced, structural disease as aortic stenosis)
Episodes of hypoglycemia could be a possible cause too, but in that case, the syncope has different characteristics, and in addition, it can be excluded, thanks to the fasting glucose measured by the patient at home and the laboratory tests that indicate an elevated HbA1c.
In this case, considering the left ventricular dysfunction and the morphologic abnormalities of the myocardium, the most probable and dangerous cause of syncope could be ventricular arrhythmias.
Indeed, during hospitalization, a non-sustained ventricular tachycardia lasting 23 s was recorded at the ECG monitoring symptomatic for dizziness and profound weakness but without syncope.
Final Diagnosis
The final diagnosis was non-sustained ventricular tachycardia symptomatic of dizziness in patient with left ventricular non-compaction and heart failure.
13.2 Left Ventricular Non-compaction
Left ventricular non-compaction (LVNC) is a rare congenital cardiomyopathy characterized by a “spongy” feature of the myocardium. A thin, compacted epicardial layer under an extensive non-compacted endocardial layer can be noticed with the common cardiac image techniques. Prominent trabeculation and deep recesses that communicate with the left ventricular cavity can be visible too. The “spongy” morphologic feature is not characteristic only of the left ventricle but can also involve the right ventricle. This is why talking about “non-compaction cardiomyopathy” seems to be more correct. The non-compaction of the myocardium is a consequence of an abnormal embryonic development of the ventricular myocardium. During the eighth week of gestation, when the interventricular septum is complete, the increase of ventricular volumes is responsible of compression against the trabeculae of the ventricular walls. This lead to the growth of the compact layer and the organization of some trabeculae in the papillary muscle.
At the same time, intramyocardial vascularization develops from the coronary arteries following an epicardial to endocardial, base to apex, and septum to side wall direction [1]. For this reason, the non-compaction morphology is more common in the apex, side, and inferior walls of the left ventricle.
Epidemiology
Genetics
Non-compaction cardiomyopathy is a heterogeneous disease from the genetic point of view which includes either familiar or sporadic forms [5]. Familiar forms are more frequent in the male sex, while sporadic forms are equally distributed in both sexes. The most common form of inheritance is autosomal dominant compared to either X-linked or autosomal recessive forms [6].
So far, have been reported mutation in several genes in patients with LVNC and the most frequent mutations involve genes encoding: proteins of the cytoskeleton, sarcomere, and mitochondria [7].
Tafazzin
Alpha-Dystrobrevin (DTNA)
Alpha-dystrobrevin is a cytoskeleton protein. A mutation in the gene coding for alpha-dystrobrevin has been identified in patients with LVNC, occasionally related to congenital heart disease [8, 11].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


