Fig. 11.1
HeartMate XVE left ventricular assist device (Permission from Thoratec Corporation)
The Thoratec VAD (Thoratec Corp) is a pneumatically powered device that is available in paracorporeal (PVAD) and intracorporeal (IVAD) versions (Figs. 11.2 and 11.3). Both devices can be utilized for uni- or bi-ventricular support. There is a hospital-based external pneumatic drive console and a portable pneumatic driver allows ambulatory outpatient support. These devices are implanted as BTT or for bridge to recovery (BTR) and received FDA approval for the BTT indication in 1995. The Thoratec device is the primary VAD used for biventricular support and does not have the size limitation associated with the Syncardia TAH. In addition, this system allows for explant in the case of myocardial recovery. The Thoratec VAD has a 65 mL stroke volume with a polyurethane blood pumping chamber and two mechanical valves. It produces a clinical beat range of 40–110 beats per minute and a flow rate of 1.3–7.2 L/min. The PVAD is positioned paracorporeally on the anterior abdominal wall and can be used in patients with a BSA of >0.75 m2. For an LVAD, the left ventricular apex is most commonly used for inflow and outflow is to the ascending aorta. For an RVAD configuration, drainage is typically from the right atrium and outflow is to the main pulmonary artery. For the IVAD, the device has a titanium housing and can be implanted either in a pre-peritoneal pocket or intraperitoneal location.



Fig. 11.2
Thoratec paracorporeal ventricular assist device (PVAD) (Permission from Thoratec Corporation)

Fig. 11.3
Thoratec intracorporeal ventricular assist device (IVAD) (Permission from Thoratec Corporation)
The SynCardia Cardio West (SynCardia Systems, Inc., Tucson, AZ) total artificial heart (TAH) (Fig. 11.4) was under development since the 1960s and the first successful implant of a TAH was performed in 1985 as BTT [11]. The SynCardia TAH is a biventricular, pneumatic pulsatile pump that replaces the native ventricles and all valves. The complete displacement of the ventricles produces a stroke volume of 70 mL per beat with a cardiac output of 7–8 L/min. The ventricles are placed in orthotopic position after suturing polyurethane inflow connectors to the right and left atrial cuffs of the patient. Dacron outflow conduits are snapped onto the mounts of the TAH ventricles after completion of the anastomoses to the great vessels. The percutaneous driveline connects to the external console that contains the pneumatic drivers. A portable driver now potentially allows patients to be discharged from the hospital. Due to the size of the device and because it is fully implanted within the pericardial cavity, careful patient selection is necessary to avoid size mismatch and potential compression of the inferior vena cava or left superior pulmonary vein. Selection criteria include: left ventricular end-diastolic diameter >70 mm, cardiothoracic ratio >0.5, computed tomography scan volume >1500 mL, and antero-posterior chest diameter (from sternum to spine) >10 cm.
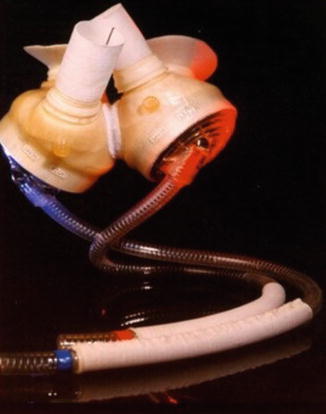

Fig. 11.4
Syncardia Cardio West total artificial heart (TAH) (Permission from Syncardia)
Continuous-Flow Left Ventricular Assist Devices
There has been a major shift to the use of continuous-flow LVADs since 2008 when the HeartMate II device was approved for the BTT indication by the United States FDA. Axial and centrifugal flow LVADs are much smaller, lighter, and more durable than the previous generation of volume displacement, pulsatile pumps (Fig. 11.5). The HeartMate II (Thoratec Corp) is an implantable axial flow LVAD that is indicated for long-term support as BTT or DT in patients with end-stage HF. This has been the most widely used implantable LVAD in recent years. The pump is typically placed in a pre-peritoneal pocket created inferior to the diaphragm, with the inflow cannula inserted at the left ventricular apex and the outflow graft anastomosed to the ascending aorta. A percutaneous driveline from the pump exits the right or the left upper quadrant of the abdomen. Other components include a microprocessor-based system controller, which is worn or carried by the patient, and controls and monitors the function of the pump. The HeartMate II can provide up to 10 L/min of flow with an operating speed range of 6000–15,000 rpm although speeds are rarely set above 10,000 rpm. Power is provided by both AC and DC power sources with portable batteries for ambulatory operation. The system monitor displays the pump speed, flow, pulsatility index (PI), and power. The monitor can be queried for device parameter history and changes in pump speed are made from this component. The HeartMate II axial flow rotary pump contains a magnet that is rotated by the electromotive force generated by the motor. Rotation of the rotor provides the force to propel blood from the left ventricle through the pump and into the ascending aorta. Pump flow depends on the rotational speed of the rotor and the pressure difference between the inlet and outlet of the pump. The device is very sensitive to afterload and hypertension must be managed appropriately. The internal pump surfaces including the rotor, inlet and outlet stators, have a smooth polished titanium surface. The inflow and outflow conduits have a textured titanium microsphere surface similar to the HeartMate XVE. These surfaces are designed to minimize thrombus development and anticoagulation is recommended for all patients with aspirin and warfarin. The BTT clinical trial for this device demonstrated a 68 % survival rate at 1 year in the initial study cohort and this has continued to improve in subsequent studies [3]. Recent institutional series have reported 1 year survival of >80 % [12]. The HeartMate II DT trial was a 2:1 randomized comparison with the HeartMate XVE LVAD in 200 patients [13]. There were 134 in the HeartMate II group and 66 in the XVE group. The actuarial survival rates of 68 % at 1 year and 58 % at 2 years were a significant improvement over the HeartMate XVE at 55 % and 24 % respectively.


Fig. 11.5
HeartMate II left ventricular assist device (Permission from Thoratec Corporation)
The HeartWare Ventricular Assist Device (HVAD) (HeartWare, Inc., Framingham, MA) is a centrifugal flow pump that is implanted in the pericardial space at the apex of the left ventricle (Fig. 11.6). The HVAD was designed for use as a long-term implantable device. A short integrated inflow cannula and the small size of the pump allow for the intra-pericardial placement without the need for developing a pump pocket. The HeartWare system consists of the HVAD pump, controller with rechargeable batteries, and the system monitor. The HVAD pump incorporates an integrated inflow cannula, a 10 mm gel-impregnated polyester outflow graft with strain relief, a percutaneous driveline, and an apical sewing ring. The strain relief prevents kinking of the outflow graft. The HVAD has a displaced volume of 50 mL and weighs 140 g. The pump can generate a flow of 10 L/min and has a pump speed operating range of 1800–4000 rpm. The impeller has integrated rotor magnets and uses a passive, noncontacting suspension system for rotation of the impeller [14]. A hermetically sealed electric motor within the pump housing generates electromagnetic fields to move the impeller with dual motor stators to create laminar flow. The frictionless movement of the impeller eliminates heat generation and wear of the components which greatly increases durability. The short integrated inflow cannula is inserted into the left ventricle, and the outflow graft connects the HVAD pump to the ascending aorta via an end-to-side anastomosis. A sewing ring secures the pump to the left ventricle. The HVAD has also been used in isolated cases as an implantable bi-VAD with separate pumps being implanted for right and left ventricular support [15]. The HVAD has been approved by the U.S. Food and Drug administration for BTT in 2013 [16] and is currently undergoing clinical trials for DT. Initial clinical experience and reports have indicated that long-term support with this device has been safe and effective with a 1 year survival rate of 86 % in a BTT population [17]. The BTT clinical trial data that was recently published demonstrated overall non-inferiority versus contemporaneously implanted, commercially available VADs at 180 days [18]. Kaplan-Meier survival at 1 year was 86 % compared to 85 % in the control group.


Fig. 11.6
Heartware HVAD (Permission from Heartware Corporation)
Timing of MCS for End-Stage Heart Failure
Continued improvement in LVAD technology and clinical outcomes has made this option available to more patients with advanced heart failure. The current generation of continuous-flow devices has significantly decreased the incidence of peri-operative and long-term complications including bleeding, adverse neurological events, infection, right heart failure, arrhythmias, and rate of hospital re-admission relative to the previous generation of pulsatile, volume displacement pumps [19]. In addition, the durability of these continuous-flow devices is far superior to the pulsatile devices with patients having been supported for up to 7 years without any device-related issues. The timing of LVAD implantation is critical to both short and long-term outcomes. The majority of patients being referred for MCS continue to be hospitalized for decompensated heart failure and are being supported with inotropes and/or an intra-aortic balloon pump (IABP). Post-operative survival and rate of discharge to home is far superior for patients who are not in critical cardiogenic shock at the time of implant [20]. For patients who are transplant candidates the timing of LVAD implant should be based on the balance of the clinical status and factors that may prolong the time on the waiting list. These include an elevated panel reactive antibody level, greater weight, and O blood type. Another important factor in the timing of LVAD implant is the inability to tolerate short-term inotropic support, typically as a result of stimulating ventricular arrhythmias. Long-term inotropic support is associated with a 1-year survival of <10 % [21] and patients who are inotrope-dependent should be considered for earlier LVAD implant if the waiting time for transplant is prolonged. In addition, patients who need a combined heart-kidney or other abdominal organ transplant will also have longer wait times due to the limitation of typically needing a local donor. Patients with these characteristics that prolong wait list time should be considered for earlier referral for MCS before they decompensate and become critically ill. As recently reported, each of the following clinical factors has a significant negative impact on 1-year survival and should be taken into consideration for patients who are being evaluated for transplant or are already listed with regard to referral for MCS: worsening renal function with creatinine >1.8 mg/dL, inability to tolerate ACE inhibitors, ARBs, or beta-blockers, diuretic dose >.5 mg/kg/day, recurrent admission for heart failure, no clinical improvement with CRT, inability to walk one block without dyspnea, and dyspnea at rest [22]. Another indication for earlier consideration of MCS is irreversible elevated pulmonary vascular resistance which is a contraindication to heart transplant, generally >4 Woods units. Pulmonary hypertension has been shown to have a favorable response to unloading with an LVAD allowing subsequent transplantation without the high risk of right ventricular failure [23].
Recent data for HeartMate II patients shows the relationship between clinical status at the time of implant and short and long-term outcomes [24]. Patients were stratified into three groups based on INTERMACS score: Group 1 (INTERMACS 1), Group 2 (INTERMACS 2 and 3), and Group 3 (INTERMACS 4–7). Boyle et al. Survival to discharge was 67.9 % for Group 1 (n = 28), 93.5 % for Group 2 (n = 49), and 95.8 % for Group 3 (n = 24). Length of stay also correlated with INTERMACS score: Group 1: 45.1 days, Group 2: 40.1 days, and Group 3: 18.3 days. One-year survival was greatest for patients in Group 3 at 95.8 % versus 73 % for Groups 1 and 2. Longer-term survival at 18 months was also significantly improved for Group 3 (95.8 %) versus Group 1 (50.2 %) and Group 2 (72.7 %). In addition, a recent study of 468 patients who underwent HeartMate II LVAD implant at 36 centers as bridge to transplant showed equivalent 30-day and 1-year survival compared to conventional cardiac transplantation (97 % and 87 % respectively) [25]. Furthermore, post-transplant survival was not found to be influenced by duration of LVAD support. The authors conclude that the improved durability and reduced short and long-term morbidity associated with the HeartMate II LVAD has reduced the need for urgent cardiac transplantation, which may have adversely influenced post-transplant survival in the pulsatile LVAD era. There is a recent trend to consider implant of most LVADs on an elective, scheduled basis. Patients are typically evaluated and accepted for LVAD therapy as BTT or DT. Most of these patients can tolerate 2–3 days off inotropic drugs and be discharged from the hospital and return home and come into the hospital the day of the surgery. This reduces the risk of nosocomial infection and other comorbidities. Some patients are clearly dependent on more aggressive medical therapy and cannot be discharged pre-LVAD. This trend is increasing as a recent poll indicated that 85 % of programs reported using this strategy with increasing practice due to good associated results.
LVAD Versus Bi-VAD or TAH
The incidence of right heart failure requiring an RVAD at the time of LVAD implant has decreased with the transition to continuous-flow devices. The reasons for this are unclear but may be related to less volume loading of the right heart by this new generation of pumps. Only 4 % of patients in the HeartMate II Bridge to transplant trial required RVAD support, however, 13 % required extended inotropic support [3]. Patients who require RVAD support at the time of LVAD implant have significantly worse survival than those with adequate right heart function [26]. In addition, planned biventricular support has much better outcomes than LVAD implant followed by RVAD implant [27]. Therefore, it is imperative to be able to identify patients who are at high risk for right ventricular failure and plan for bi-VAD or TAH support. The University of Michigan group has recently developed a right ventricular failure risk score (RVFRS) as a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates [28]. Right ventricular failure was defined as the need for RVAD implant, inotropic support for >14 days, or hospital discharge on an inotrope. Of 197 LVAD implants, 68 (35 %) were complicated by post-operative RV failure. These LVAD implants were primarily pulsatile devices with only 15 % being continuous-flow pumps. A vasopressor requirement (4 points), aspartate aminotransferase ≥80 IU/I (2 points), bilirubin ≥2.0 mg/dL (2.5 points), and creatinine ≥2.3 mg/dL (3 points) were independent predictors of RV failure. The odds ratio for RV failure for patients with an RVFRS ≤3.0, 4.0–5.0, and ≥5.5 were 0.49 (95 % CI 0.37–0.64), 2.8 (1.4–5.9) and 7.6 (3.4–17.1), respectively, and 180-day survival was 90 ± 3 %, 80 ± 8 %, and 66 ± 9 % for each group (Fig. 11.7). Fitzpatrick and colleagues identified risk factors for requiring an RVAD at the time of LVAD implant based on 266 patients who underwent LVAD placement at the University of Pennsylvania between 1995 and 2007 [29]. Of these patients, 99 (37 %) required RVAD support. The most significant predictors for RVAD support at the time of LVAD implant were cardiac index ≤2.2 L/min/m2, RV stroke work index ≤0.25 mmHg • L/m2, severe pre-operative RV dysfunction, creatinine ≥1.9 mg/dL, previous cardiac surgery, and systolic blood pressure ≤96 mmHg. Each of these criteria that are met is assigned a score of 1 or 0 if they are or are not met, and a risk score was derived from the following equation: 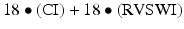 . The maximum possible score is 98 and a score of ≥50 was predictive of the need for bi-VAD support with a sensitivity and specificity of 83 % and 80 %, respectively. In our experience at the University of Chicago Medical Center with >120 continuous-flow LVAD implants, the ratio of mean pulmonary artery pressure to right atrial pressure has been very predictive of the degree of RV dysfunction and potential need for an RVAD. When this ratio is ≥2, none of the patients required RVAD support, however, this ratio was <2 for the patients who did require an RVAD at the time of HeartMate II LVAD implant (n = 5). It is our observation that higher pulmonary artery pressures are associated with better RV function.
. The maximum possible score is 98 and a score of ≥50 was predictive of the need for bi-VAD support with a sensitivity and specificity of 83 % and 80 %, respectively. In our experience at the University of Chicago Medical Center with >120 continuous-flow LVAD implants, the ratio of mean pulmonary artery pressure to right atrial pressure has been very predictive of the degree of RV dysfunction and potential need for an RVAD. When this ratio is ≥2, none of the patients required RVAD support, however, this ratio was <2 for the patients who did require an RVAD at the time of HeartMate II LVAD implant (n = 5). It is our observation that higher pulmonary artery pressures are associated with better RV function.
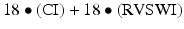 . The maximum possible score is 98 and a score of ≥50 was predictive of the need for bi-VAD support with a sensitivity and specificity of 83 % and 80 %, respectively. In our experience at the University of Chicago Medical Center with >120 continuous-flow LVAD implants, the ratio of mean pulmonary artery pressure to right atrial pressure has been very predictive of the degree of RV dysfunction and potential need for an RVAD. When this ratio is ≥2, none of the patients required RVAD support, however, this ratio was <2 for the patients who did require an RVAD at the time of HeartMate II LVAD implant (n = 5). It is our observation that higher pulmonary artery pressures are associated with better RV function.
. The maximum possible score is 98 and a score of ≥50 was predictive of the need for bi-VAD support with a sensitivity and specificity of 83 % and 80 %, respectively. In our experience at the University of Chicago Medical Center with >120 continuous-flow LVAD implants, the ratio of mean pulmonary artery pressure to right atrial pressure has been very predictive of the degree of RV dysfunction and potential need for an RVAD. When this ratio is ≥2, none of the patients required RVAD support, however, this ratio was <2 for the patients who did require an RVAD at the time of HeartMate II LVAD implant (n = 5). It is our observation that higher pulmonary artery pressures are associated with better RV function.
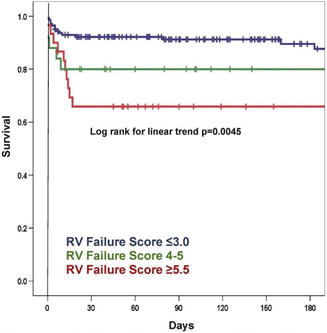
Fig. 11.7
Kaplan-Meier survival curve for each RV failure risk score strata. The 180-day post-left ventricular assist device survival curves for each score strata are displayed. RV right ventricular (Permission from Matthews et al. [28])
The typical hemodynamic scenario during weaning from cardiopulmonary bypass with severe RV dysfunction is poor LVAD flow, elevated right atrial pressure, and low pulmonary artery and systemic blood pressures. TEE shows poor RV function and dilation with bowing of the interventricular septum to the left. Inotropic support should be initiated prior to weaning from bypass and inhaled NO can be utilized as an adjunctive therapy in the setting of RV dysfunction. If two high dose inotropes are required to achieve adequate hemodynamics, consideration should be given to short-term RVAD support. At our center, we have primarily used the Levitronix CentriMag device as an RVAD due to its ease of use and cost-effectiveness. Weaning of the RVAD can be done at the bedside and is usually able to be removed within 3–5 days of implant using low-moderate dose inotropic support.
For transplant candidates with severe biventricular dysfunction and very high risk scores for RV failure following LVAD implant, the Thoratec PVAD/IVAD, SynCardia total artificial heart, and ABIOMED AB 5000 are the current options for long-term biventricular support as a bridge to transplant. These devices are currently FDA approved for discharge to home.
Destination Therapy
It is estimated that 250,000 patients in the United States are in the terminal phase of systolic heart failure and are suffering from severe symptoms that are refractory to maximal medical therapy [1]. Heart transplantation remains the best long-term solution for this population, but is available to only a very small fraction of these patients due to the extremely limited number of donor organs available and many are not suitable candidates for transplant due to other co-morbidities. Long-term mechanical circulatory support with a pulsatile LVAD was shown to be superior at 1 and 2-years versus optimal medical therapy in the landmark REMATCH trial which was published in 2001 for patients ineligible for cardiac transplant [10]. This was the first trial to evaluate an LVAD as destination therapy (DT) and also demonstrated a marked improvement in functional capacity and quality of life. The enthusiasm for DT was tempered by the 2-year survival rate of only 23 % versus 8 % with medical therapy and a high post-operative mortality. The recent results of the HeartMate II destination therapy trial show significantly better survival rates, device durability, and lower incidence of device-related complications [13]. One and 2-year survival was 68 % and 58 % respectively, which is far superior compared to the pulsatile device utilized in the REMATCH trial.
Candidate selection and timing of LVAD implant are critical for achieving excellent outcomes in the destination therapy patient population. The most common indication for DT LVAD implant versus listing for heart transplantation is age. Other co-morbidities that may be a contraindication to listing for transplant include significant end-organ dysfunction, treated malignancy within the past 5 years, severe pulmonary hypertension (PVR >5 Woods units), peripheral vascular disease, obesity (BMI >35), substance abuse, and psychosocial factors. Patients with any of these issues who are refractory to optimal medical therapy should be considered for an LVAD as destination therapy. Renal failure and pulmonary dysfunction may be relative contraindications for DT, however, the experience with continuous-flow devices and chronic dialysis is very limited and long-term results have not been published to date. Lietz and co-authors developed a risk score for in-hospital mortality following pulsatile LVAD implantation for DT by studying 309 patients who were underwent implant in the post-REMATCH period between 2002 and 2005 at 66 hospitals [30]. Overall survival on LVAD support was 86.1 %, 56.0 %, and 30.9 % at 30 days, 1 year, and 2 years. The following predictors of 90-day in-hospital mortality after LVAD implantation were identified by multivariable analysis: platelet count ≤148,000, serum albumin ≤3.3 g/dL, international normalized ratio >1.1, vasodilator therapy at time of implantation, mean pulmonary artery pressure ≤25.3 mmHg, aspartate aminotransferase >45 U/dL, hematocrit ≤34 %, blood urea nitrogen >51 U/dL, and lack of intravenous inotropic support. The risk factors and scoring are likely to be somewhat different in the current era of continuous-flow LVADs but are very useful to estimate the risk of operative mortality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


