Left-to-Right Shunts
“A surgeon knows how to operate, a good surgeon knows when to operate, and an excellent surgeon know when not to operate”
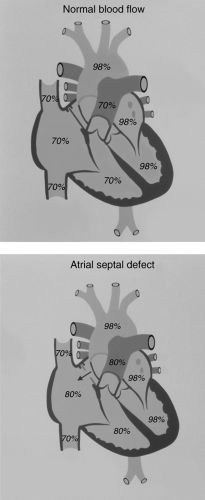
A left-to-right shunt exists when blood from the left atrium, left ventricle, or aorta transits to the right atrium or its tributaries, the right ventricle, or the pulmonary artery. Because blood in the left atrium, left ventricle, and the aorta is normally fully oxygenated, left-to-right shunt lesions result in fully oxygenated blood recirculating through the lungs, a rather inefficient situation for gas exchange. Hence, the lungs receive all the deoxygenated blood from the systemic venous return (this is equal to cardiac output or systemic blood flow [Qs]) plus the volume of fully oxygenated blood that “short circuits” to the right side of the heart through the defect that allows the left-to-right shunt.
The magnitude of a left-to-right shunt can be expressed in terms of the ratio (Qp/Qs) of the volume of pulmonary flow (Qp) and systemic flow (Qs). Normally, this ratio is 1 because the volume of blood that is pumped to the lungs (Qp) is equal to the volume of blood that is pumped to the body (Qs). For patients with a left-to-right shunt, Qp/Qs will be >1. In general, Qp/Qs <1.5 is considered a small shunt, Qp/Qs = 1.5 to 2 is considered a moderate shunt, and Qp/Qs >2 is considered a large shunt. Qp/Qs can be calculated if the blood oxygen saturation is known for the mixed venous, pulmonary arterial, left atrial, and aortic blood (see Table 9.1).
The presence of a left-to-right shunt results in a volume overload of one or more cardiovascular chambers or structures. The chamber or structure that is volume overloaded depends upon the location of the anatomic defect causing the left-to-right shunt. With an atrial septal defect (ASD), blood passes from the left to right atrium. Hence, the left atrium, right atrium, right ventricle, and pulmonary artery are volume overloaded. After the blood transits the lungs and reaches the left atrium, some of the it again short circuits to the right atrium (by passing through the ASD), but a normal volume of blood goes through the mitral valve to reach the left ventricle. Therefore, the left ventricle is not volume overloaded.
In the presence of ventricular septal defect (VSD), blood passes from the left ventricle to the pulmonary artery. Technically, the blood transits the right ventricle, but because the right and left ventricle are contracting simultaneously, the right ventricle does not realize a
volume overload in this situation. The right atrium also does not realize a volume overload. However, the pulmonary artery is receiving an increased volume of blood as is the left atrium and left ventricle.
volume overload in this situation. The right atrium also does not realize a volume overload. However, the pulmonary artery is receiving an increased volume of blood as is the left atrium and left ventricle.
TABLE 9.1 Formula to Calculate the Ratio of Pulmonary and Systemic Blood Flow | ||
|---|---|---|
|
In the presence of patent ductus arteriosus (PDA), blood passes from the aorta to the pulmonary artery. Therefore, the pulmonary artery, left atrium, and left ventricle are volume overloaded but the right atrium and right ventricle are not.
The presence of a left-to-right shunt can result in pressure and volume overload of one or more cardiovascular chambers or structures. The presence of pressure overload will depend on the location and size of the left-to-right shunt and the pulmonary vascular resistance. ASDs that are not associated with increased pulmonary vascular resistance result in only a mild elevation of right ventricular (RV) and pulmonary artery pressure. A large VSD (i.e., one that is larger than the aorta) will result in elevated RV and pulmonary artery pressures that are equal to the pressure in the left ventricle. A small PDA will not cause significant elevation of the RV pressure, but a large PDA can cause elevated RV and pulmonary artery pressures equal to the pressure in the aorta.
Atrial Septal Defects
Incidence, Types, and Embryology
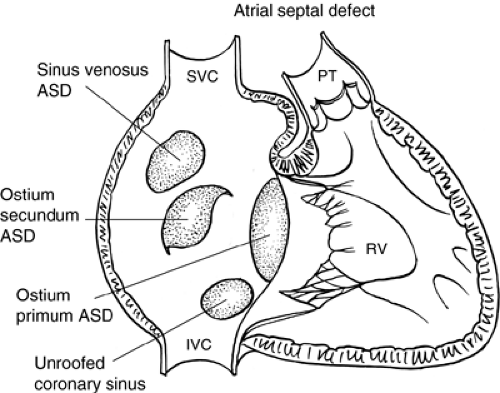
ASDs occur in 1 of 1,500 live births and constitute 6% to 10% of congenital cardiac defects. There is a female-to-male predominance of 2:1. There are four types of ASDs: (i) Ostium secundum, (ii) ostium primum, (iii) sinus venosus, and (iv) unroofed coronary sinus (see Figs. 9.1 and 9.2). There have been rare cases of familial ASD that have been associated with the genes encoding the transcription factors TBX5, NKX2.5, and GATA4, as well as the structural protein MYH6.
The most common form of ASD is the ostium secundum. This occurs in the region of the fossa ovalis. It results from excessive absorption of the septum primum or insufficient development of the septum secundum or both.
Ostium primum ASD is a type of atrioventricular sepal defect. It is located in the inferior aspect of the atrial septum contiguous with the tricuspid and mitral valves. It results from lack of closure of the ostium primum by the endocardial cushions. Because the endocardial cushions also form major portions of the mitral and tricuspid valves, it is not surprising that abnormalities of the atrioventricular (AV) valves are associated with ostium primum ASDs. A cleft in the septal leaflet of the mitral valve invariably is associated with ostium primum ASDs.
Sinus venosus ASDs occur in the posterosuperior aspect of the atrial septum. Frequently, there is associated partial anomalous drainage of the right upper pulmonary vein. This
vein can drain into the superior vena cava (SVC) or the right atrium. It is speculated that resorption of the wall between the vena cava and pulmonary veins results in the ASD. This also explains the anomalous drainage of the right upper pulmonary vein into the right atrium or SVC, commonly associated with sinus venous ASDs.
vein can drain into the superior vena cava (SVC) or the right atrium. It is speculated that resorption of the wall between the vena cava and pulmonary veins results in the ASD. This also explains the anomalous drainage of the right upper pulmonary vein into the right atrium or SVC, commonly associated with sinus venous ASDs.
The most uncommon form of ASD is the unroofed coronary sinus. The coronary sinus is in apposition to the posterior aspect of the left atrium, but the orifice is in the right atrium. If
a hole exists in the roof of the coronary sinus, the coronary sinus and the left atrium will be in continuity, and, therefore, the right and left atria will be in communication with each other.
a hole exists in the roof of the coronary sinus, the coronary sinus and the left atrium will be in continuity, and, therefore, the right and left atria will be in communication with each other.
Clinical Presentation
Most patients with ASD are asymptomatic and seek medical attention when a heart murmur is detected. Frequently, the murmur is not appreciated to be a pathologic murmur until the child is as old as 3 to 5 years. In less than 10% of patients with ASD, symptoms of congestive heart failure (CHF) and growth failure can occur in infancy. If the ASD is associated with another defect such as PDA, CHF is much more common and the defect becomes apparent early in life.
Physical Examination
In patients with ASD, the RV impulse felt along the lower left sternal border or the subxiphoid area may be more forceful than normal. The first heart sound will be normal. The second heart sound will be more widely split than normal and does not become single with expiration, the so-called fixed splitting of S2. There are several murmurs that can be heard in patients with ASD. All patients with ASD have a systolic ejection murmur, which is best heard along the left sternal border and is loudest at the upper left sternal border. This murmur is caused by excessive blood flow through the pulmonary valve, is similar to an “innocent pulmonary flow murmur,” and probably explains why many patients with ASD are not diagnosed until several years of age. With moderate and large defects, a middiastolic murmur can be heard along the lower right or left sternal border. In some patients, this murmur is best heard over the xiphoid and is caused by excessive blood flow through
the tricuspid valve. This murmur is not heard in patients with a small ASD. Generally, the Qp/Qs must be >1.5 for this murmur to be heard. In a patient with ASD, this murmur does not result from blood flowing through the ASD itself.
the tricuspid valve. This murmur is not heard in patients with a small ASD. Generally, the Qp/Qs must be >1.5 for this murmur to be heard. In a patient with ASD, this murmur does not result from blood flowing through the ASD itself.
Patients with ostium primum ASD, in addition to the murmurs described in the preceding text, may have a murmur of mitral insufficiency because of the cleft in the septal leaflet of the mitral valve associated with this defect.
Electrocardiographic Features
The electrocardiographic (ECG) features of ASD depend on the size of the defect and the type of ASD. For secundum, sinus venosus ASDs, and the unroofed coronary sinus, the ECG can be normal if the defect results in only a small left-to-right shunt. The ECG may have an rSR’ pattern in the right precordial leads that also is frequently found in healthy individuals. For patients with moderate to large defects resulting in moderate to large left-to-right shunts, the ECG will show evidence of right atrial and RV hypertrophy and right axis deviation. There is a rare form of autosomal dominant inherited secundum ASD that is associated with first-degree AV block.
Ostium primum ASDs can be distinguished from other types of ASDs electrocardiographically because, like other forms of endocardial cushion defects, they are characterized by an initial counterclockwise frontal plane loop and left axis deviation.
Chest X-ray
The findings on chest x-ray are helpful in judging the size of the left-to-right shunt in patients with ASD. With a small shunt, the chest x-ray will be normal. As the shunt increases in size, the chest x-ray will show increased heart size and increased pulmonary vascular markings. The chest x-ray is not helpful in distinguishing the various types of ASD.
Echocardiographic and Cardiac Catheterization Issues
The diagnosis of ASD and its type can be confirmed by echocardiography. In addition, the degree of right atrial and RV enlargement and hypertrophy can be assessed. Elevation of pulmonary artery pressure can be approximated using Doppler techniques. It can be difficult to diagnose sinus venosus defects with transthoracic echocardiography, and, for some patients, transesophageal echocardiography may be necessary to confirm this diagnosis. However, a clue to the presence of a sinus venosus ASD, using transthoracic echocardiography, is increased blood flow in the SVC that results from the anomalous connection of the right upper pulmonary vein that is frequently associated with sinus venosus ASD.
In the era of echocardiography, it is rarely necessary to perform cardiac catheterization for the diagnosis of ASD. However, cardiac catheterization is now used to deliver and implant devices to close secundum ASDs without the need for open heart surgery.
Treatment
The treatment of ASD involves surgical or device closure. For secundum ASD, surgical closure can be accomplished by direct suture or patch closure and device closure by cardiac catheterization techniques. There are several types of devices to close secundum ASDs. It is important that a rim of septal tissue be present around the entire circumference of the defect to stabilize the device. Currently, device closure is the preferred method for closing
secundum ASD in appropriately selected patients. However, the long-term outcome of this treatment remains unknown.
secundum ASD in appropriately selected patients. However, the long-term outcome of this treatment remains unknown.
For ostium primum ASD, patch closure is invariably used, and in most cases, the cleft in the mitral valve leaflet is repaired. For sinus venosus ASD, the anomalous drainage of the right upper pulmonary vein is corrected, and the ASD is closed.
The usual age for closure of an uncomplicated ASD is 2 to 4 years. In rare cases of infants with ASD and heart failure, surgery should be performed during infancy.
Endocarditis prophylaxis is recommended for all types of ASDs except ostium secundum.
Natural History of Atrial Septal Defect and Outcome of Treatment
With the exception of ostium secundum, ASDs do not spontaneously close. It has been estimated that in children, up to 15% of ostium secundum ASDs close by 4 years of age.
Untreated ASDs, over time, can produce RV enlargement, fibrosis, and failure. They are associated with atrial enlargement, which, with time, can result in atrial arrhythmias. The presence of ASD can allow paradoxical embolization. Lastly, in some patients, there can be an association between ASD and pulmonary vascular obstructive disease. However, whether the ASD produces pulmonary vascular disease or whether the pulmonary vascular disease results in stretching of a patent foramen ovale is not clear.
The risk of death following surgical closure of an uncomplicated ASD is <1%. Murphy et al. reported a 25- to 30-year follow-up of patients who had undergone surgery for ostium secundum or sinus venosus ASD at the Mayo Clinic from 1956 to 1960. There were four perioperative deaths, and all these patients were older than 46 years and had pulmonary hypertension. Long-term survival of patients who had undergone surgery at <24 years of age was similar to that of the general population. Patients who had undergone surgery at >24 years of age had poorer survival than an age-matched control.
El-Najdawi et al. assessed the outcome of 334 patients who had undergone surgery for ostium primum ASD at the Mayo Clinic between 1956 and 1995. The surgical mortality was 3%; ten-year survival was 96%. Reoperation, usually for mitral valve malfunction or subaortic stenosis, was necessary for 38 patients.
Ventricular Septal Defects
Incidence, Types, and Embryology
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree