20 Left Atrial Isomerism
I. CASE
A. Fetal echocardiography findings
1. The findings suggest a picture of left atrial isomerism (LAI) or polysplenia syndrome (Table 30-1).
a. There is dextrocardia (cardiac axis = 60 degrees), and the stomach is on the right side of the fetal abdomen as well.
b. There is a midline liver and multilobed right-sided spleen.
c. The heart is mildly enlarged (cardiothoracic ratio = 0.47).
d. The inferior vena cava (IVC) is interrupted, with azygous continuation to a right superior vena cava (SVC) that connects directly to the right-sided atrium.
e. There is a left SVC to coronary sinus to right-sided atrium.
f. The four-chamber view reveals absence of the crux of the heart consistent with an atrioventricular septal defect (AVSD), with severe disproportion between the ventricles.
g. There is a dominant right-sided left ventricle (LV).
2. The outflow assessment reveals normally related great arteries with mild asymmetry. Aorta–to–pulmonary artery diameter ratio is 1:0.7.
3. The aortic valve is in continuity with the common atrioventricular (AV) valve and arises from the LV. The size is normal but the velocity through the aorta is increased (2 m/s). There is also mild aortic insufficiency.
4. The pulmonary artery arises from a small right ventricle (RV) that is composed largely of an outlet chamber. The pulmonary valve is bicuspid, with mild flow acceleration by Doppler (2 m/s).
5. The branch pulmonary arteries are a good size for gestational age (right pulmonary artery = 1.9 mm, left pulmonary artery = 2.2 mm).
6. The aortic arch is leftward, and the ductal and aortic arches have antegrade flow.
7. Interatrial flow is unrestricted through a primum atrial septal defect (ASD) with a bidirectional shunt.
8. The pulmonary veins connect via a confluence that joins the posterior wall of the left-sided atrium, and they exhibit a normal flow pattern.
9. The hepatic veins connect to the right-sided atrium via a common trunk.
10. There is fetal bradycardia with an atrial heart rate of 115 bpm and a ventricular heart rate of 68 bpm, and complete AV block is suspected.
11. The LV Tei index (myocardial performance index) is normal.
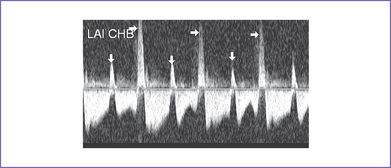
12. There was systolic a wave reversal at the atrial rate on each beat in the ductus venosus with intermittently larger a waves, suggesting cannon waves occurring during ventricular systole (Fig. 20-1).
13. There is no evidence of hydrops.
14. The peripheral arterial Doppler has a normal pulsatility index of 1.2, and the middle cerebral artery pulsatility index is 2.0.
TABLE 20-1 FEATURES CHARACTERIZING NORMAL ASYMMETRY

Adapted from Yoo SJ, Hornberger LK, Smallhorn J: Abnormal visceral and atrial situs and congenital heart disease. In Yagel S, Silverman NH, Gembruch U (eds): Fetal Cardiology: Embryology, Genetics, Physiology, Echocardiographic Evaluation, Diagnosis and Perinatal Management of Cardiac Diseases. London: Taylor & Francis Group, 2002.
D. Fetal management and counseling
a. Because the mother has already had chorionic villous sampling, there was no further discussion about fetal karyotype except that aneuploidy and 22q11.2 deletion are extremely rare in isomerism.
b. Diagnosis of tetralogy of Fallot (TOF) should prompt referral for the following:
E. Delivery
1. In LAI with complete heart block and complex congenital heart disease, delivery should be at term or as near as possible in a tertiary care center.
2. If the baby is developing fetal hydrops, earlier delivery may be necessary, as long as it is clearly possible to improve the hemodynamic condition of the fetus with delivery and intervention.
3. If delivery with postnatal intervention is not believed to be effective, aside from termination of pregnancy if desired by the patient, letting nature take its course with progressive hydrops may be the best option, with close follow-up of the mother to be certain there is no harmful consequence for her.
4. If there is no evolution of hydrops, cesarean section delivery may be considered given the difficulties with fetal monitoring in fetal bradycardia. Ideally, a planned cesarean section is performed at 36 to 38 weeks of gestation.
F. Neonatal management
b. Prostaglandin E1 (PGE1) infusion is indicated if there is evidence of critical pulmonary outflow tract obstruction with retrograde ductal flow in utero or unless the direction of ductal flow is not certain.
d. At times, volume and inotropic support may be indicated to improve ventricular function.
e. Assessment of heart rate in relation to the baby’s clinical condition.
G. Follow-up
1. Subacute bacterial endocarditis (SBE) prophylaxis is required for life.
2. For residual hemodynamic abnormalities, anticongestive medications and some restriction of activities may be required.
3. The patient should have one or two outpatient follow-up visits each year, with 12-lead electrocardiogram (ECG) and echocardiogram.
4. If there is any history of unexplained palpitations or abnormal heart rhythm, a metabolic stress test with 24-hour Holter monitoring is recommended.
5. Pacemaker checks are necessary to detect lead or battery failure.