Fig. 18.1
Double orifice repair: the edge-to-edge technique, shown here, is used to repair large segments of redundant mitral leaflet. This is especially applicable to central mitral valve leaks
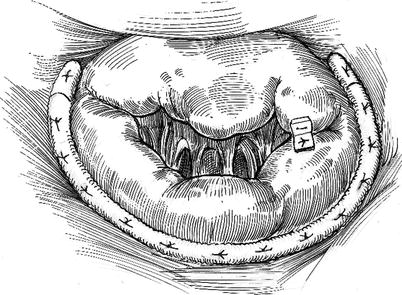
Fig. 18.2
Para-commissural repair: the edge-to-edge technique, shown here, is used to repair prolapsing valve regions that are either near or at the commissures
Repair Indications
The edge-to-edge technique has been used for the correction of mitral regurgitation due to many different etiologies and mechanisms. After an over 15 years’ experience, we believe that the main indications of this procedure are:
Bileaflet prolapse (Barlow’s disease)
Anterior leaflet prolapse (when it is difficult to visualize the subvalvular apparatus)
Commissural prolapse
Functional mitral regurgitation (ischemic or idiopathic dilated cardiomyopathy)
Leaflet retraction with contralateral “pseudo-prolapse” (i.e. over-riding anterior leaflet)
Endocarditis with leaflet prolapse or a flail segment
SAM (systolic anterior motion of the anterior leaflet) not responding to volume loading and drug induced beta blockade.
Operative Conduct
Surgical Access
As mentioned before, the edge-to-edge technique is effective either using a standard sternotomy or mini-invasive approaches (mini-thoracotomy and robotic surgery). When a median sternotomy is used normothermic cardiopulmonary bypass is established by standard aortic and bi-caval venous cannulation. Wide inter-atrial groove dissection provides excellent left atrial and MV exposure. After aortic clamping, myocardial protection is assured by intermittent antegrade cold-blood cardioplegia. In the presence of aortic regurgitation retrograde cardioplegia is added.
In patients with preserved LV function and no significant comorbidities, minimally invasive methods (mini-thoracotomy or robotic surgery) are preferred. Using the latter access techniques, patients are intubated for single lung ventilation with a double-lumen endotracheal tube. For complete venous drainage a 14 F cannula is passed percutaneously through the right jugular vein and into the superior vena cava. Cardiac access is provided through a 6- to 8-cm fourth intercostal space mini-thoracotomy with minimal rib spreading. A lateral port is placed to introduce a 5-mm videoscope with a side arm to fill the operative field with CO2. After incising the pericardium, trans-thoracic pericardial sutures are retracted through the chest wall for inter-atrial groove and left atrial exposure. Femoral arterial and venous cannulation are performed and cardiopulmonary bypass (CPB) is established at 28–30 °C.
For minimally invasive operations cardiac arrest is achieved by using the Chitwood trans-thoracic aortic clamp (Scanlan International, Minneapolis, MN), inserted through the lateral chest wall in the second or third intercostal space. Myocardial protection is provided by intermittent aortic root blood cardioplegia. A trans-thoracic atrial retractor, placed in the fourth intercostal space lateral to the right sternal border, is used for mitral valve exposure. The valve is repaired using either long shafted instruments and video-assistance or robotic methods.
For robotic repairs the camera port is placed in the fourth intercostal space 2–3 cm lateral to the mid-clavicular line. After inspecting the intra-thoracic anatomy and alignment for optimal MV visualization, a 2–4 cm working port is made in the same interspace several centimeters lateral to the camera port. The left robotic arm port is placed in the third intercostal space 2–3 cm anterior to the anterior axillary line. The right robotic arm port is placed in the fifth or sixth intercostal space slightly anterior to the mid-axillary line. The dynamic left atrial retractor is placed through a port either in the fourth or the fifth intercostal space in the mid-clavicular line.
It is mandatory that operating room 2D/3D TEE studies to plan every mitral reconstruction. Necessary images include four-chamber views, as well as short and long-axis two-chamber views, both obtained at mid-esophageal and deep trans-gastric levels. Right and left ventricular function is assessed by obtaining, short-axis, mid-chamber views. Obviously, post-operative evaluations must confirm the presence of MV competence, satisfactory trans-valvular flow characteristics, and preserved ventricular function.
Operative Technique
After the prolapsing or flail segment of one or both leaflets is identified, the free edge of the diseased leaflet is anchored to the corresponding edge of the opposing leaflet with a 4-0 polypropylene suture. As previously described, a ‘double orifice’ or a ‘para-commissural repair’ is performed depending on the central or commissural localization of the diseased leaflet portion. For a proper ‘double orifice repair, it is crucial to identify correctly the middle portion of leaflets to avoid valve distortion that could result in residual leakage. With mitral regurgitation, secondary to Barlow’s disease, the intra-operative analysis must define important redundancies of all valve tissue, including those related to multiple scallops on both anterior and posterior leaflets. With severe bileaflet prolapse there is usually a significant elongation of the chordal apparatus (Fig. 18.3). Here, the central portion of both anterior and posterior leaflets must be identified, using the subvalvular apparatus as a guide. Using a nerve hook, the chordae connected to the anterolateral and posteromedial papillary muscles are defined. The convergence of the two chordal groups is the ‘anatomical middle’ of each leaflet.
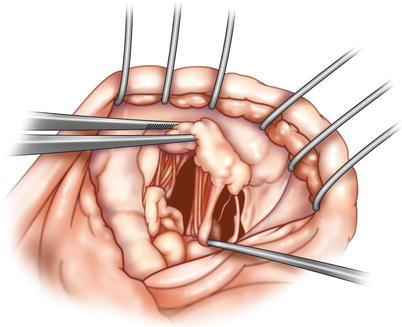

Fig. 18.3
Bileaflet prolapse—anterior leaflet “center” determined: the “anatomic middle” of the anterior leaflet is identified in the region where chordae from both papillary muscles merge on the free edge. If there is significant posterior leaflet prolapse, a similar determination is necessary
After the ‘anatomical middle’ has been identified, a double-armed 4-0 polypropylene “stay stitch” is passed through the central part of A2 and P2 in the leaflet rough zone (Fig. 18.4). Thereafter, symmetry of the two orifices created is checked immediately using forceps (Fig. 18.5). Also, left ventricular saline filling helps determine the adequacy of the two orifices and well as coaptation symmetry and valve competence (Fig. 18.6). Both leaflets already should appear to be symmetric and competent. Thereafter, deep running suture bites are taken along 1–2 cm of A2 and P2, completing the edge-to-edge approximation. We suggest placement of a mattress suture line followed by an “over and over” second closure suture line. All stitches are placed deep through leaflet rough zones to reduce tissue redundancy. As a general rule, the width of the suture line should be minimized to reduce the risk of valvular stenosis. After reconstruction, the residual mitral area can be measured by introducing Hegar valve dilators into each orifice (Fig. 18.7). A global valve area of more than 2.5 cm2 is considered acceptable for ‘normal size’ patients (two 18-mm Hegar dilators correspond to an area greater than 2.5 cm2). A ring annuloplasty is recommended to complete and stabilize the repair. For mini-invasive surgery an incomplete ring also can be used to save implantation time. Final competence is evaluated by forceful saline filling of the left ventricle. This procedure is done both before and after ring implantation. In most instances the valve will be competent even before the ring is implanted.
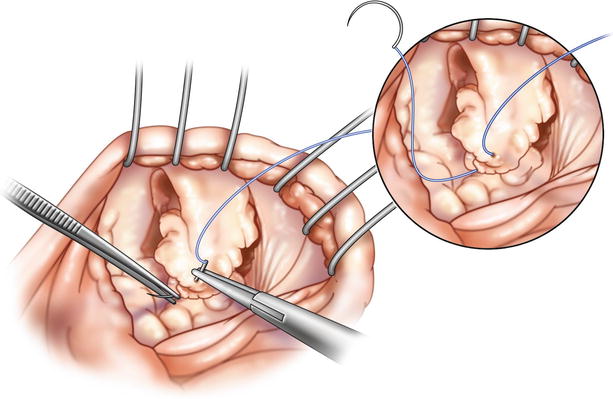
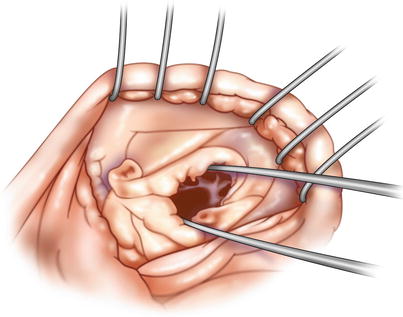
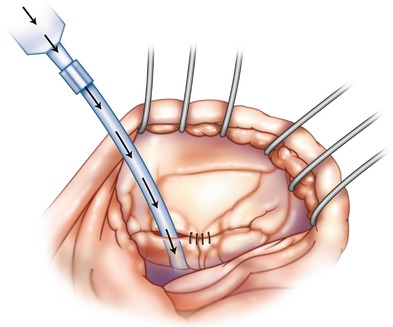

Fig. 18.4
Anterior and posterior leaflet edge approximation: first 4-0 polypropylene “Stay” suture is passed in a standardized manner along the free edge of the middle scallop of the anterior and posterior leaflets. Pledgets are not necessary

Fig. 18.5
Individual orifice inspection: after a central “stay” suture has been placed between the anterior and posterior leaflet, each orifice should be inspected with surgical forceps to be sure that there is an adequate opening

Fig. 18.6




Saline valve testing: the valve is tested for competence by the rapid infusion of saline into the ventricle. This should be done both after the “stay” suture has been placed as well as after the approximating suture line has been completed. We place a silastic tube that has been attached to a bulb syringe across the line of coaptation. This test should provide a good idea of leaflet coaptation and symmetry as well as valve competence
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


