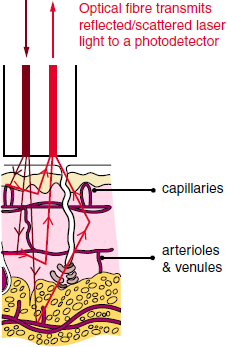
CHAPTER 4 Blood flow is a fundamental property of cellular, tissue, and organ metabolism. Homeostasis of skeletal microcirculation is central to bone metabolism, calcium balance, fracture repair, bony response to stress, and the osseous integration of implantable prostheses. Additionally, pathological alteration in normal skeletal blood flow has important clinical consequences. While osteonecrosis of the hip is a well-known ischemic phenomenon leading to degenerative joint disease, osteoarthritis may have an ischemic origin as well.13 Laser Doppler flowmetry (LDF) is a technique developed over 30 years ago to allow invasive and non-invasive measurement of blood flow within tissue. Initially used by Stern to measure blood flow in human skin and rodent renal tissues, the technology has demonstrated ease of use and reproducible results for both real-time dynamic and steady state monitoring of biological tissues when compared to other methods of blood flow measurement.33, 34, 39 Its development and application has been detailed by Swiontkowski, an early proponent of LDF use in orthopaedic surgery.37 LDF relies on the Doppler shift phenomenon to measure relative changes in microvascular circulation. Light is emitted by a helium-neon gas or nearinfrared semiconductor laser that is absorbed and scattered by the tissues under the probe (Fig. 4.1). Back-scattered (reflected) light is measured by one or more photodetectors. Movement of red blood cells within the microcirculation under the field of study leads to alterations in the signal amplitude being reflected back to the photodetector(s). The alteration in the frequency of the signal represents the Doppler shift and allows for signal processing to occur. (Moor Instruments [Internet]) Fredriksson et al. provides an excellent in-depth review of the theory behind LDF.6 Swiontkowski et al. (1986) demonstrated utility of the technique and offered comparison with established methods of blood flow measurement, concluding that easy-to-use LDF had several advantages over established microsphere techniques.39 Standefer et al. evaluated the ability of four different techniques to detect ischemic events in juvenile pigs. When comparing LDF to fiber optic pressure, piezoelectric pressure, and the partial pressure of oxygen, they found that all methods recognized ischemic events. However, the time required for 50% reduction in oxygenation differed, with LDF noted to be the fastest although subject to motion artifact.31 LDF measurements are relative and measured in blood perfusion units, or flux, which is proportional to the velocity and concentration of RBCs within the tissues studied. No constant or baseline values have been identified for human tissues; therefore, LDF is best used to measure the response of the microcirculation in a tissue under varying physiological circumstances. Vegter’s 1991 canine work sought to demonstrate a relationship between increased intracapsular pressure and decreased hip blood flow. While LDF flux values were shown to be highly reproducible, with increased pressure impacting femoral head blood flow in both juvenile and adult dogs, this study further demonstrated the great variability in LDF flux measurements within the same animal and between animals.40 Multiple devices and probes have been developed for different applications. With current manufacturers including Perimed (www.perimedinstruments.com), Moor (us.moor.co.uk), and BioPac (www.biopac.com), the specific probe design allows for unique tissue measurements. Probes may be invasive or non-invasive and often are custom designed for the intended application. Exact positioning and stability is vital to decrease motion artifact caused by the instrument, observer, or subject. As the probes can be highly sensitive to movement of the user, patient, or device, a critical effort at limiting the in situ movement of a given probe is essential to obtaining quality data for analysis and is facilitated through specialized stands, holders, guides, or sutures. Probes may be used with direct application or through endo- or arthroscope portals. Bone probes (Fig. 4.2) generally require a pre-drilled hole to decrease motion artifact. Long term implantable probes are also available for use.23 Limitations in the penetration, absorption, and reflection of laser light as well as probe design and signal processing generally result in an area of study limited to less than 2mm depth and a 2.5 mm area. In the orthopaedic literature, LDF has been frequently used to evaluate the changes in blood flow to the femoral head under varying physiologic and iatrogenic stresses. In the adult, most blood supply to the femoral head comes from the medial femoral circumflex artery which ascends on the ventral side of the short external rotators and pierces the hip capsule posteriorly and inferiorly on the femoral neck.3, 43 Perforating vessels then enter the hip joint capsule to supply the femoral head with blood. This tenuous blood supply is at risk of disruption during femoral neck fracture and surgical or traumatic hip dislocation, and secondary to any process resulting in a hip joint effusion which itself may cause a pressure-based occlusion of the terminal penetrating arterioles supplying oxygenation to the femoral head. Fig. 4.2. Standard bone probe, VP7BS, Moor Instruments. Diameter is 3.3 mm, standard needle length is 100 mm, with a range of 30–150 mm. us.moor.co.uk/i/products/66/157_xl.png. Reprinted with permission.17 LDF offers a minimally invasive way to monitor blood supply throughout a surgical procedure. Before any surgical manipulation, a baseline measurement of red blood cell flux must be obtained. Age, systemic illness limiting perfusion (e.g., lupus, sickle cell anemia, and inflammatory vasculitis), vasoactive drugs, environmental conditions, type of anesthesia, and hemodynamic status can influence baseline values. Prior studies have demonstrated that values differ between and within patients to such an extent that comparisons are possible only in the relationship of normal to pathologic within the same patient.25, 38 Comparison of the baseline measurement to a measurement at any point throughout the surgical procedure can alert the surgeon to any changes in femoral head microcirculation which may require further action. The Swiontkowski group also demonstrated early clinical utility of LDF while evaluating osteonecrosis of the femoral head.35 This work provided a mechanism for direct measurement of subchondral bone blood flow and demonstrated that articular cartilage, due to its low blood flow, did not impede penetration of laser Doppler. Although results within patients had a wide variation, they were reproducible and measurements in areas of collapse were found to be significantly lower than normally perfused areas in the same patients.38 Beck’s group found that increased intracapsular pressure in human subjects caused loss of pulsatile LDF signal, which subsequently returned with aspiration of joint, suggesting that monitoring before, during, and after decompression of fracture hematoma or other effusion will demonstrate adequate decompression and return of femoral head blood flow.4 In cases of planned rotational proximal femoral osteotomy for avascular necrosis (AVN) of the femoral head, ensuring that the intact articular surface is at least 1/3 of the total head area is important for successful outcomes. Standard studies, including x-ray, CT scan, and MRI are not sensitive in this regard. In a series by Fukuoka, these modalities failed to demonstrate the full extent of a lesion up to 25% of the time. The margin of necrotic tissue was unable to be identified by MRI in 5% of patients.7 Laser speckle, a form of flowmetry, was used as an adjunct to MRI to increase the accuracy of necrotic area size determination. Necrotic margins were evident in a subset of hips (approximately 5% of those studied) that were not revealed with MRI or surgical evaluation of the collapse. A major downside to laser speckle, compared to other forms of LDF, is that dislocation of the femoral head was necessary to obtain the flow map. The effect of surgical approach to the hip on femoral head perfusion has been evaluated. Khan’s group looked at femoral head blood flow following posterolateral and posterior (transgluteal) approaches. Using cefuroxime concentration in bone as an indirect indicator, their data indicated significantly more damage to blood flow with posterolateral approach.12 Additional study demonstrated decreased blood flow following posterior approach for hip resurfacing arthroplasty; however, intraoperative comparison to the anterolateral approach using LDF failed to demonstrate significant differences.19 This work is in contrast to more recent work which used LDF to demonstrate up to a 40% reduction in blood flow with the posterior approach.1 A modified posterior approach for hip resurfacing was shown to preserve femoral head oxygenation using a gas electrode.32 The clinical relevance of these studies is questionable as follow-up study demonstrated no significant difference in blood flow at one year using SPECT-CT scan in a small sample size.2 Zlotorowicz et al. (2013) demonstrated the development of AVN in 10 out of 35 patients with a fracture/dislocation of the hip, two of whom were without any blood flow to the femoral head following reduction as assessed by CT angiography.43
LASER DOPPLER FLOWMETRY
4.1 Introduction
4.2 Physiology
4.3 Devices
4.4 Clinical Applications
4.4.1 Fractures and osteotomies
4.4.2 Surgical approaches
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree