Beta-blocker therapy is recommended after ST-segment elevation acute myocardial infarction (STEMI) in current guidelines, although its efficacy in those patients who have undergone primary percutaneous coronary intervention (PCI) has not been adequately evaluated. Of 12,824 consecutive patients who underwent sirolimus-eluting stent implantation in the J-Cypher registry, we identified 910 patients who underwent PCI within 24 hours from onset of STEMI. Three-year outcomes were evaluated according to use of β blockers at hospital discharge (349 patients in β-blocker group and 561 patients in no–β-blocker group). Patients in the β-blocker group more frequently had hypertension, low left ventricular ejection fraction (LVEF), a left anterior descending artery infarct, and statin use than those in the no-β-blocker group. No difference was observed between the β-blocker and no–β-blocker groups in mortality (6.6% vs 6.6%, p = 0.85; propensity score adjusted hazard ratio 1.10, 95% confidence interval 0.64 to 1.90, p = 0.70) or in incidence of major adverse cardiac events (all-cause death, recurrent myocardial infarction, and heart failure hospitalization, 13.5% vs 12.1%, p = 0.91; hazard ratio 1.13, 95% confidence interval 0.76 to 1.66, p = 0.53). Better outcomes were observed in the β-blocker group than in the no–β-blocker group in a subgroup of patients with LVEF ≤40% (n = 125, death 6.4% vs 17.4%, p = 0.04; major adverse cardiac events 14.5% vs 31.8%, p = 0.009). In conclusion, β-blocker therapy was not associated with better 3-year clinical outcomes in patients with STEMI who underwent primary PCI and had preserved LVEF.
Randomized controlled trials and meta-analyses have demonstrated beneficial effects of β blockers on survival in patients with ST-segment elevation acute myocardial infarction (STEMI). Based on the results of these studies, current American College of Cardiology/American Heart Association guidelines for treatment of STEMI recommend daily oral administration of β blockers to hemodynamically stable patients who have no contraindications to β blockers. However, it is less clear whether β-blocker therapy improves long-term clinical outcomes in patients who have undergone primary percutaneous coronary intervention (PCI) after STEMI. In this study, the long-term effect of β-blocker use was investigated in consecutive patients who underwent primary PCI after STEMI in patients enrolled in the j-Cypher Registry.
Methods
The study design and patient enrollment for the j-Cypher Registry have been described in detail elsewhere. In brief, the j-Cypher Registry is a physician-directed prospective multicenter registry in Japan enrolling consecutive patients undergoing sirolimus-eluting stent implantation without any exclusion criteria. Although data entry was basically left to the individual sites, clinical research coordinators in the data management center (Department of Cardiology, Kyoto University Hospital, Kyoto, Japan) supported data entry when necessary. Logical inconsistencies were resolved by inquiries to the site investigators and/or by audits against the original data sources. Follow-up data were obtained from hospital charts or by contacting patients and/or referring physicians at 30 days, 6 months, 1 year, and yearly thereafter. When death, MI, and stent thrombosis (ST) were reported, the events were adjudicated using the original source documents by a clinical events committee.
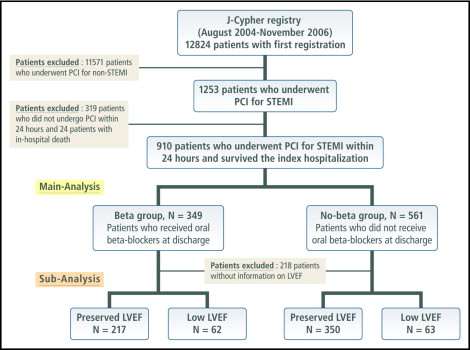
The present post hoc subanalysis of the j-Cypher Registry was intended to evaluate the efficacy of oral β blockers in patients with STEMI who have undergone primary PCI in real-world clinical practice. Of 12,824 patients enrolled in the j-Cypher Registry from August 2004 to November 2006, 1,253 patients had an admission diagnosis of STEMI. PCI was performed within 24 hours from onset in 934 patients (74.5%), from 24 hours to 7 days in 169 patients (13.5%), and after ≥7 days in 150 patients (12.0%). Patients who did not undergo PCI within 24 hours from onset and 24 patients with in-hospital death were excluded from the present analysis. Therefore, the present study population consisted of 910 patients who underwent primary PCI within 24 hours after onset of STEMI and were discharged alive from the index hospitalization ( Figure 1 ). We classified patients into the β-blocker group (those who received β blockers at discharge, n = 349, 38.4%) and the no–β-blocker group (those who did not receive β blockers at discharge, n = 561, 61.6%). Left ventricular ejection fraction (LVEF) measured by echocardiography was reported in 692 patients (76.0%). Subgroup analysis was also conducted in these 692 patients with known LVEF.
The relevant review boards in all 37 participating centers approved the study protocol. Written informed consent was obtained from all patients enrolled. The study sponsor was not involved in the study design; in the collection, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication.
The primary outcome measurement for the present analysis was all-cause death. The secondary outcome measurement was major adverse cardiac events (MACEs) defined as a composite of all-cause death, recurrent MI, and heart failure hospitalization. Other end points assessed included cardiac death, sudden death, recurrent MI, ST, stroke, heart failure hospitalization, target lesion revascularization, and any coronary revascularization.
During follow-up, death was regarded as cardiac in origin unless obvious noncardiac causes could be identified. MI was adjudicated according to the definition in the Arterial Revascularization Therapy Study. Heart failure hospitalization was defined as hospitalization due to exacerbation of heart failure adjudicated by local investigators. ST was defined according to the Academic Research Consortium definition. Definite ST assessed on an individual patient basis was used as the end point for ST. Stroke was defined as symptomatic cerebral infarction or intracranial bleeding necessitating hospitalization. Low LVEF was defined as ≤40% and preserved LVEF was defined as >40%. Presence of chronic kidney disease was defined by an estimated glomerular filtration rate <30 ml/min/1.73 m 2 according to the Modification of Diet in Renal Disease study equation modified for Japanese patients.
Continuous variables are presented as mean ± SD, and categorical variables are expressed as number and percentages. Categorical variables were compared with chi-square test when appropriate; otherwise, Fisher’s exact test was used. Continuous variables were compared with t test or Wilcoxon rank-sum test based on distribution. Cumulative incidences of clinical event rates were estimated by the Kaplan-Meier method and differences were assessed with log-rank test. Because use of β blockers was decided by physicians, we developed a propensity score for use of β blockers based on variables that were associated with their use. Potential variables assessed for associations using β blockers by univariate analysis were as follows: age ≥80 years, male gender, body mass index <25.0 kg/m 2 , hypertension, diabetes mellitus, current smoking, chronic kidney disease, previous MI, previous stroke, peripheral vascular disease, heart failure, previous PCI, previous coronary artery bypass graft, infarct-related coronary artery location, total stent length >28 mm, reference diameter before PCI <2.5 mm, use of intravascular ultrasound, multivessel stenting, and use of statins. We also evaluated the association of LVEF with use of β blockers, but we replaced this with heart failure if the association was significant because we wanted to avoid the loss of samples due to missing data for LVEF. We developed a propensity score from the multivariable logistic model with variables with significant associations for use of β blockers by univariate analysis. We then developed a Cox proportional hazard model with variables of use of β blockers and the decile propensity scores. Results of multivariable analysis are expressed as adjusted hazard ratios and their 95% confidence intervals of use of β blockers for the primary and secondary outcome measurements. Subgroup analyses were also performed in patients with preserved LVEF and low LVEF. All analyses were conducted by physicians (NO and TK) and a statistician (TM) using JMP 7 and SAS 9.2 (SAS Institute, Cary, North Carolina). All reported p values were 2-sided and p values <0.05 were regarded as statistically significant.
Results
The proportion of patients for whom β blockers were prescribed at hospital discharge after primary PCI within 24 hours from onset of STEMI was 38.3% and varied widely according to institutions (range 0% to 100%, median 42.5%). Patients in the β-blocker group more frequently had hypertension, low LVEF, a left anterior descending coronary artery infarct, statin use, and intravascular ultrasound use than those in the no–β-blocker group. Current smokers were more prevalent in the β-blocker group. Prevalences of diabetes mellitus, previous stroke, and previous MI were not different between the 2 groups ( Table 1 ). There were 6 factors that were associated with β-blocker use, namely hypertension, current smoking, low LVEF, left anterior descending coronary artery-related infarction, use of statins, and use of intravascular ultrasound. Because there were missing data for LVEF, we substituted it with heart failure, and used these 6 factors to develop the propensity score thereafter.
| Variable | All Patients (n = 910) | β-Blocker Group (n = 349) | No–β-Blocker Group (n = 561) | p Value |
|---|---|---|---|---|
| Age (years) | 67.4 ± 11.6 | 66.4 ± 11.5 | 68.0 ± 11.6 | 0.05 |
| Age ≥80 years | 146 (16%) | 51 (14%) | 95 (16%) | 0.35 |
| Men | 694 (76%) | 264 (75%) | 430 (76%) | 0.73 |
| Body mass index <25.0 kg/m 2 | 615 (67%) | 232 (66%) | 383 (68%) | 0.52 |
| Hypertension | 622 (68%) | 256 (73%) | 366 (65%) | 0.01 |
| Diabetes mellitus | 349 (38%) | 144 (41%) | 205 (36%) | 0.16 |
| Current smoker | 346 (38%) | 148 (42%) | 198 (35%) | 0.03 |
| Chronic kidney disease | 46 (5%) | 22 (6%) | 24 (4%) | 0.18 |
| Hemodialysis | 16 (1%) | 9 (2%) | 7 (1%) | 0.14 |
| Previous myocardial infarction | 80 (8%) | 28 (8%) | 52 (9%) | 0.52 |
| Previous stroke | 79 (8%) | 27 (7%) | 52 (9%) | 0.42 |
| Peripheral vascular disease | 51 (5%) | 18 (5%) | 33 (5%) | 0.64 |
| Heart failure | 158 (17%) | 63 (18%) | 95 (16%) | 0.67 |
| Previous percutaneous coronary intervention | 120 (13%) | 41 (11%) | 79 (14%) | 0.31 |
| Previous coronary artery bypass surgery | 13 (1%) | 6 (1%) | 7 (1%) | 0.56 |
| Left ventricular ejection fraction | 52.3 ± 12.1 | 51.0 ± 11.9 | 53.2 ± 12.2 | 0.02 |
| Left ventricular ejection fraction ≤40% | 125 (18%) | 62 (22%) | 63 (15%) | 0.02 |
| Infarct-related artery location | 0.007 | |||
| Left anterior descending coronary artery | 415 (46%) | 186 (53%) | 229 (41%) | |
| Left circumflex coronary artery | 117 (13%) | 42 (12%) | 75 (13%) | |
| Right coronary artery | 361 (40%) | 114 (33%) | 247 (44%) | |
| Left main coronary artery | 11 (1%) | 5 (1%) | 6 (1%) | |
| Saphenous vein graft | 5 (0.5%) | 2 (0.6%) | 3 (0.5%) | |
| Artery graft | 1 (0.1%) | 0 (0%) | 1 (0.2%) | |
| Treatment procedures | 0.16 | |||
| Sirolimus-eluting stent | 511 (56%) | 201 (57%) | 310 (55%) | |
| Bare metal stent | 352 (39%) | 125 (36%) | 227 (40%) | |
| Balloon angioplasty | 47 (5%) | 23 (7%) | 24 (4%) | |
| Total stent length >28 mm | 188 (21%) | 78 (23%) | 110 (20%) | 0.39 |
| Reference diameter before PCI <2.5 mm | 300 (34%) | 110 (32%) | 190 (35%) | 0.49 |
| Use of intravascular ultrasound | 457 (51%) | 208 (60%) | 249 (45%) | 0.0001 |
| Multivessel stenting | 550 (60%) | 203 (58%) | 347 (61%) | 0.27 |
| Medication at discharge | ||||
| Aspirin | 902 (99%) | 347 (99%) | 555 (98%) | 0.72 |
| Thienopyridine | 865 (95%) | 331 (94%) | 534 (95%) | 0.82 |
| Angiotensin-converting enzyme inhibitors | 228 (25%) | 93 (26%) | 135 (24%) | 0.38 |
| Angiotensin receptor blockers | 465 (51%) | 185 (53%) | 280 (49%) | 0.36 |
| Statins | 497 (54%) | 223 (63%) | 274 (48%) | 0.0001 |
Cumulative incidence of death at 3 years was 6.6% for the entire study population. No difference was observed in 3-year mortality between patients in the β-blocker group and those in the no–β-blocker group ( Figure 2 , Table 2 ). Adjusted hazard ratios of use of β blockers for 3-year mortality was 1.10 (95% confidence interval 0.64 to 1.90, p = 0.70). Cumulative incidence of MACEs at 3 years was 12.6% for the entire study population. No difference was observed in 3-year MACE between patients in the β-blocker group and those in the no–β-blocker group ( Figure 2 , Table 2 ). Adjusted hazard ratios of use of β blockers for 3-year MACEs was 1.13 (95% confidence interval 0.76 to 1.66, p = 0.53).

| Event | All Patients (n = 910) | β-Blocker Group (n = 349) | No–β-Blocker Group (n = 561) | Log-Rank p Value |
|---|---|---|---|---|
| Death | 60 (6.6%) | 23 (6.6%) | 37 (6.6%) | 0.85 |
| Cardiac death | 27 (3.0%) | 9 (2.6%) | 18 (3.2%) | 0.53 |
| Sudden death | 8 (0.9%) | 3 (0.9%) | 5 (0.9%) | 0.92 |
| Reinfarction | 24 (2.6%) | 9 (2.6%) | 15 (2.7%) | 0.85 |
| Stent thrombosis (definite) | 16 (1.8%) | 7 (2.0%) | 9 (1.6%) | 0.70 |
| Stroke | 32 (3.5%) | 13 (3.7%) | 19 (3.4%) | 0.88 |
| Heart failure hospitalization | 61 (6.7%) | 26 (7.5%) | 35 (6.2%) | 0.56 |
| Target lesion revascularization | 133 (14.6%) | 52 (14.9%) | 81 (14.4%) | 0.93 |
| Any revascularization | 247 (27.1%) | 93 (26.6%) | 154 (27.4%) | 0.61 |
| Major adverse cardiac events | 115 (12.6%) | 47 (13.5%) | 68 (12.1%) | 0.71 |
Baseline characteristics in the low and preserved LVEF subgroups are presented in Tables 3 and 4 , respectively. In 125 patients with low LVEF, mortality and MACEs in the β-blocker group were significantly lower than those in the no–β-blocker group ( Figures 3 and 4 , Table 5 ). However, in 567 patients with preserved LVEF, there were no differences in mortality and MACE between the 2 groups ( Figures 3 and 4 , Table 6 ).
| Variable | All Patients (n = 125) | β-Blocker Group (n = 62) | No–β-Blocker Group (n = 63) | p Value |
|---|---|---|---|---|
| Age (years) | 68.7 ± 11.1 | 67.3 ± 11.5 | 70.2 ± 10.7 | 0.15 |
| Age ≥80 years | 25 (20%) | 9 (14%) | 16 (25%) | 0.13 |
| Men | 100 (80%) | 51 (49%) | 49 (50%) | 0.53 |
| Body mass index <25.0 kg/m 2 | 89 (71%) | 46 (74%) | 43 (69%) | 0.55 |
| Hypertension | 92 (73%) | 46 (74%) | 46 (73%) | 0.88 |
| Diabetes mellitus | 54 (43%) | 28 (45%) | 26 (42%) | 0.66 |
| Current smoker | 50 (40%) | 29 (46%) | 21 (33%) | 0.12 |
| Chronic kidney disease | 6 (4%) | 4 (6%) | 2 (3%) | 0.44 |
| Hemodialysis | 2 (1%) | 2 (3%) | 0 (0%) | 0.24 |
| Previous myocardial infarction | 17 (13%) | 7 (8%) | 10 (8%) | 0.45 |
| Previous stroke | 15 (12%) | 7 (7%) | 8 (7%) | 0.81 |
| Peripheral vascular disease | 11 (8%) | 5 (5%) | 6 (9%) | 0.77 |
| Heart failure | 41 (32%) | 18 (20%) | 23 (36%) | 0.37 |
| Previous percutaneous coronary intervention | 25 (20%) | 10 (12%) | 15 (12%) | 0.28 |
| Previous coronary artery bypass surgery | 2 (1%) | 2 (3%) | 0 (0%) | 0.24 |
| Left ventricular ejection fraction | 34.8 ± 5.3 | 33.9 ± 5.8 | 35.8 ± 4.6 | 0.04 |
| Infarct-related artery location | 0.05 | |||
| Left anterior descending coronary artery | 85 (68%) | 48 (77%) | 37 (59%) | |
| Left circumflex coronary artery | 8 (6%) | 4 (6%) | 4 (6%) | |
| Right coronary artery | 30 (24%) | 10 (16%) | 20 (32%) | |
| Left main coronary artery | 2 (2%) | 0 (0%) | 2 (3%) | |
| Saphenous vein graft | 0 (0%) | 0 (0%) | 0 (0%) | |
| Artery graft | 0 (0%) | 0 (0%) | 0 (0%) | |
| Treatment procedures | 0.35 | |||
| Sirolimus-eluting stent | 71 (57%) | 39 (63%) | 32 (51%) | |
| Bare metal stent | 46 (37%) | 19 (31%) | 27 (43%) | |
| Balloon angioplasty | 8 (6%) | 4 (6%) | 4 (6%) | |
| Total stent length >28 mm | 35 (29%) | 16 (26%) | 19 (32%) | 0.51 |
| Reference diameter before PCI <2.5 mm | 39 (32%) | 15 (25%) | 24 (39%) | 0.12 |
| Use of intravascular ultrasound | 81 (65%) | 50 (80%) | 31 (50%) | 0.0003 |
| Multivessel stenting | 73 (58%) | 33 (53%) | 40 (63%) | 0.24 |
| Medication at discharge | ||||
| Aspirin | 123 (98.4%) | 62 (100%) | 61 (96%) | 0.50 |
| Thienopyridine | 117 (93.6%) | 60 (96%) | 57 (90%) | 0.27 |
| Angiotensin-converting enzyme inhibitors | 24 (19.2%) | 16 (25%) | 8 (12%) | 0.06 |
| Angiotensin receptor blockers | 69 (55.2%) | 32 (51%) | 37 (58%) | 0.42 |
| Statins | 62 (49.6%) | 39 (62%) | 23 (36%) | 0.003 |
| Variable | All Patients (n = 567) | β-Blocker Group (n = 217) | No–β-Blocker Group (n = 350) | p Value |
|---|---|---|---|---|
| Age (years) | 67.0 ± 11.8 | 66.7 ± 11.7 | 67.2 ± 11.8 | 0.59 |
| Age ≥80 years | 87 (15%) | 34 (15%) | 53 (15%) | 0.87 |
| Men | 426 (75%) | 161 (74%) | 265 (75%) | 0.68 |
| Body mass index <25.0 kg/m 2 | 376 (66%) | 142 (65%) | 234 (67%) | 0.69 |
| Hypertension | 381 (67%) | 156 (71%) | 225 (64%) | 0.06 |
| Diabetes mellitus | 200 (35%) | 87 (40%) | 113 (32%) | 0.06 |
| Current smoker | 212 (37%) | 89 (41%) | 123 (35%) | 0.16 |
| Chronic kidney disease | 28 (4%) | 11 (5%) | 17 (4%) | 0.91 |
| Hemodialysis | 9 (1%) | 4 (1%) | 5 (1%) | 0.73 |
| Previous myocardial infarction | 50 (8%) | 17 (7%) | 33 (9%) | 0.51 |
| Previous stroke | 47 (8%) | 16 (7%) | 31 (8%) | 0.53 |
| Peripheral vascular disease | 29 (5%) | 11 (5%) | 18 (5%) | 0.97 |
| Heart failure | 80 (14%) | 32 (14%) | 48 (13%) | 0.73 |
| Previous percutaneous coronary intervention | 68 (12%) | 22 (10%) | 46 (13%) | 0.28 |
| Previous coronary artery bypass surgery | 8 (1%) | 3 (1%) | 5 (1%) | 0.35 |
| Left ventricular ejection fraction | 56.2 ± 9.5 | 55.4 ± 9.5 | 56.7 ± 9.5 | 0.12 |
| Infarct-related coronary artery location | 0.19 | |||
| Left anterior descending | 240 (42%) | 106 (49%) | 134 (38%) | |
| Left circumflex | 78 (14%) | 27 (12%) | 51 (15%) | |
| Right | 242 (43%) | 82 (38%) | 160 (46%) | |
| Left main | 4 (0.7%) | 1 (0.5%) | 3 (1%) | |
| Saphenous vein graft | 2 (0.4%) | 1 (0.5%) | 1 (0.3%) | |
| Artery graft | 1 (0.2%) | 0 (0%) | 1 (0.3%) | |
| Treatment procedures | 0.12 | |||
| Sirolimus-eluting stent | 323 (57%) | 124 (57%) | 199 (57%) | |
| Bare metal stent | 213 (38%) | 76 (35%) | 137 (39%) | |
| Balloon angioplasty | 31 (5%) | 17 (8%) | 14 (4%) | |
| Total stent length >28 mm | 106 (19%) | 46 (22%) | 60 (18%) | 0.24 |
| Reference diameter before PCI <2.5 mm | 179 (32%) | 70 (33%) | 109 (32%) | 0.76 |
| Use of intravascular ultrasound | 338 (60%) | 142 (66%) | 196 (57%) | 0.04 |
| Multivessel stenting | 334 (58%) | 123 (56%) | 211 (60%) | 0.40 |
| Medication at discharge | ||||
| Aspirin | 561 (98%) | 215 (99%) | 346 (98%) | 0.99 |
| Thienopyridine | 536 (94%) | 202 (93%) | 334 (95%) | 0.24 |
| Angiotensin-converting enzyme inhibitors | 122 (21%) | 55 (25%) | 67 (19%) | 0.08 |
| Angiotensin receptor blockers | 303 (53%) | 118 (54%) | 185 (52%) | 0.72 |
| Statins | 329 (58%) | 140 (64%) | 189 (54%) | 0.01 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


