Fig. 11.1
Joseph Priestley (1733–1804). (From the National Portrait Gallery, by permission)
While Priestley’s discoveries in the area of oxygen and a number of other important gases are naturally the focus of this article, it should be made clear at the outset that most of his writings were in an entirely different area. First and foremost he was a minister of religion with a lifelong emphasis on a humanist philosophy that resulted in him being a fierce nonconformist. This led to severe criticism from the established church in England. He was an ardent liberal who strongly supported both the French and American revolutions, and it was these political attitudes that ultimately led to his undoing in England, and his subsequent emigration to America. By far the largest part of Priestley’s writings are on theology, and for example, in the definitive biographies such as those of Schofield [15, 16], Priestley’s work on the respiratory gases occupies only a small section. He was also a man of prodigious energy, and apart from his studies of religion and oxygen and other gases, he made important contributions to the topic of electricity, particularly its history. He produced some 50 books and when once asked how many he had written he replied “Many more, Sir, than I should like to read” [8].
Not surprisingly, there is a large literature on Priestley, especially in relation to his work on oxygen. The chief purpose of this article is to give graduate and medical students (and perhaps their mentors) some insight into the color and excitement of his discoveries. In addition it is revealing to compare Priestley with his contemporary, Lavoisier, because of their very different personalities and writings. Finally there is a discussion of Priestley as a product of the Enlightenment that had such an enormous influence in Europe in the eighteenth century and beyond, that resulted in Priestley’s interactions with Benjamin Franklin (1706–1790) and Thomas Jefferson (1743–1826), and that still resonates with many of us today.
11.2 Brief Biography
Priestley was born near Leeds, a major city in Yorkshire in the north of England. The family was rather poor but strongly religious, albeit not in conformity with the established Church of England. Priestley showed precociousness from an early age and, as noted by several biographers, at the age of four he could flawlessly recite all 107 questions and answers of the Westminster Shorter Catechism. As an example, question 1 was “What is the chief end of man?” with the answer “Man’s chief end is to glorify God, and to enjoy him forever”. This cerebral, analytical attitude was to remain with Priestley for the whole of his life.
Priestley studied theology at the Daventry Academy where liberal, enlightened emphases were strong. As a non-conformist, or dissenter from the established Church of England, he was precluded from attending most higher institutions including Oxford and Cambridge. In Daventry he developed his belief in tolerance, abhorrence of dogma, and a passion for the rational analysis of the natural world. Later Priestley moved to the prestigious Warrington Academy where he tutored in languages because of his broad knowledge of French, Italian and German, and his liberal leanings increased. He wrote an English grammar [9] that still makes enjoyable reading with its rigorous analytical style. He also produced several essays on the importance of a liberal education with an emphasis on its development. Another strong interest was electricity, particularly its history, and he published a 700-page book titled “The History and Present State of Electricity” [10] which went through five editions, and translations into French and German. He made several discoveries in this area including the conductivity of charcoal, and he proposed that electrical force was similar to Newton’s gravitational force in following an inverse square law.
Priestley then returned to Leeds where he became the minister of the Mill Hill Chapel. There he wrote his book “Institutes of Natural and Revealed Religion” [12] which further emphasized his liberal views and set out his conviction that religious beliefs should be consistent with a scientific view of the world. As a result he challenged a number of basic Christian principles and this evoked a great deal of savage criticism. Priestley questioned the divinity of Christ and the Virgin Birth, and he was one of the founders of the Unitarian Church in both England and America.
Priestley’s financial position was never particularly secure and in 1772 he entered the services of Lord Shelburne, who was immensely wealthy and had a large country estate in Wiltshire and a house in London. Shelburne stated that he needed a tutor for his children, a librarian, and an assistant, but in fact the duties were light and some of Priestley’s best experimental work on oxygen was carried out at this time. The country estate known as Bowood House is a grand English stately home that can be visited today.
In 1780 Priestley moved to Birmingham where he continued his teaching and research. While he was there he became a member of the famous Lunar Society that included inventors, scientists, manufacturers and others who met together once a month at the time of the full moon to discuss their work. The many members included the engineer James Watt, the physician and philosopher Erasmus Darwin, and the botanist William Withering known for his work on the foxglove and digitalis. However Priestley increasingly faced resistance because of his extreme liberal views. This came to a head in July of 1791 when a dinner was arranged to celebrate the anniversary of the storming of the Bastille in Paris, and this provoked a riot. A mob torched Priestley’s house destroying all of his belongings including his scientific equipment (Fig. 11.2). Priestley and his family eventually escaped to London where he lived for a period in the district of Hackney.

Fig. 11.2
Attack on Priestley’s house by a riot mob in 1791. (From the Susan Lowndes Marques Collection, by permission)
The criticism surrounding Priestley became so virulent that in 1794 he sailed with his family to America where they initially lodged in Philadelphia, the capital at that time. Before leaving England he wrote “I cannot refrain from repeating again, that I leave my native country with real regret, never expecting to find anywhere else society so suited to my disposition and habits” [8]. While Priestley was on the high seas in 1794, his contemporary, Lavoisier, was executed by guillotine in Paris. Priestley was offered a professorship at the University of Pennsylvania but declined. The family eventually moved to Northumberland County in Pennsylvania where Priestley’s son and others purchased 300,000 acres. Priestley built a new house and laboratory and this is now a national monument. However his scientific work never completely recovered from his rejection in England. Priestley died in 1804 and is buried in Riverview Cemetery in Northumberland.
11.3 First Production of Oxygen
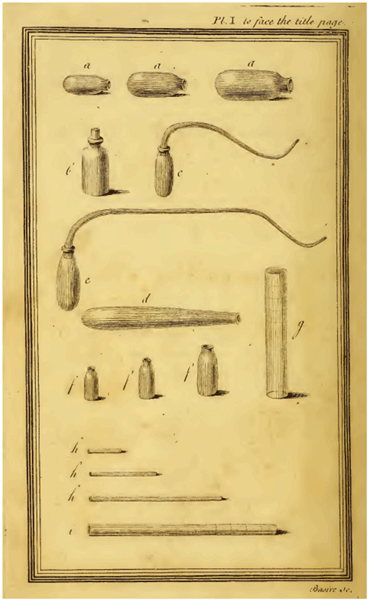
August 1, 1774 is a red letter day for respiratory physiologists because this was when Priestley first produced oxygen. He did this by heating red mercuric oxide (known at the time as mercurius calcinatus per se) by focusing the sun’s rays using a convex lens of 12 inches diameter. The experiment was not trivial. First in order to produce red mercuric oxide it was necessary to heat mercury exposed to air to near its boiling point for several months. Next was the problem of collecting the gas released from the mercuric oxide when it was heated to a very high temperature. Priestley did this by using glass containers shown in Fig. 11.3. He placed some of the red mercuric oxide in the bottom of the container, filled it with mercury, and then inverted it over a basin of mercury. Stephen Hales (1677–1761) had previously used glass containers filled with water inverted over a water bath for collection of gases [5]. When Priestley heated the mercuric oxide by focusing the sun onto it he found that a gas was evolved causing the mercury in the glass vessel to fall.
He then collected a sample of this gas (which he called dephlogisticated air) and subjected it to several tests. First he found that it was insoluble in water. This was important because he had previously produced a gas that had some of the same properties but was water-soluble. This was nitric oxide (NO) which oxidized to nitrogen dioxide (NO2). Next he placed a candle in the new gas and reported in his immortal statement “but what surprized me more than I can well express, was, that a candle burned in this air with a remarkably vigorous flame … I was utterly at a loss how to account for it” (Fig. 11.4). He also reported that “a piece of red-hot wood sparkled in it … and was consumed very fast”.
Because it is interesting to see the actual printed version, Fig. 11.4 reproduces the passage in Priestley’s chapter from his book “Experiments and Observations on Different Kinds of Air” Volume II published in 1776 [14]. This is frequently quoted as the first record of the discovery. However Fig. 11.5 reproduces the first actual statement in print. The circumstances of this are interesting. Very soon after his first experiment on August 1, 1774, Priestley set out with his patron, Lord Shelburne, for a tour of the European continent. In October they were in Paris and met with members of the Académie des Sciences, and also had dinner with Lavoisier. During this Priestley told Lavoisier about his extraordinary results, and in retrospect it was perhaps strange that he would reveal these to one of his potential competitors before he had published them. However Priestley did not believe in secrecy. In the account of his discovery [14] he wrote “As I never make the least secret of any thing that I observe, I mentioned this experiment also, as well as those with the mercurius calcinatus, and the red precipitate, to all my philosophical acquaintance in Paris, and elsewhere, having no idea at that time, to what these remarkable facts would lead”. Conversely Lavoisier put a great deal of importance on secrecy and precedence, and was guilty at times of not citing the work of others whose work he had exploited. In the event Lavoisier was quick to repeat Priestley’s experiment before the latter could return to England and continue his work.

Fig. 11.5
Priestley’s first description of his production of oxygen in a letter to Sir John Pringle. From [13]
Priestley was back in England in November 1774 but did not return to working on the new gas until March 1, 1775. In the meantime he worked on other gases that he had discovered. However on March 8 Priestley decided to test the effects of the new gas on a mouse. He found to his delight that whereas the animal could live only a quarter of an hour in common air, it survived for an hour in his new gas on two different occasions. Furthermore when the mouse was taken out it out was still quite vigorous. At this point Priestley realized the importance of getting the new work into print and on March 15 he wrote a letter to Sir John Pringle, the President of the Royal Society. This was published later in 1775 and an extract is reproduced in Fig. 11.5. Note that Priestley reiterated the effect of the gas on a burning candle and a piece of red-hot wood but he also went on to describe its effect on a mouse.
Priestley then speculated in his typical engaging way that his new gas might be useful for patients of lung disease. He stated “From the greater strength and vivacity of the flame of a candle, in this pure air, it may be conjectured, that it might be peculiarly salutary to the lungs in certain morbid cases, when the common air would not be sufficient to carry off the phlogistic putrid effluvium fast enough” (Fig. 11.6). He continued with another wonderful paragraph “My reader will not wonder, that, after having ascertained the superior goodness of dephlogisticated air by mice living in it, and the other tests above mentioned, I should have the curiosity to take it myself. I have gratified that curiosity, by breathing it, drawing it through a glass siphon, and, by this means, I reduced a large jar full of it to the standard of common air. The feeling of it to my lungs was not sensibly different from that of common air; but I fancied that my breast felt peculiarly light and easy for some time afterwards. Who can tell but that, in time, this pure air may become a fashionable article in luxury. Hitherto only two mice and myself have had the privilege of breathing it” (Fig. 11.6).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree