Fig. 9.1
Joseph Black (1728–1799). (From http://en.wikipedia.org/wiki/File:Joseph_Black_b1728.jpg)
Black’s pioneering work on carbon dioxide assures him a major place in the history of physiology. However he did much more. In fact historians of science sometimes ignore his work on carbon dioxide and instead emphasize his major advances in the area of heat. He was the first person to recognize latent heat, that is the heat added or lost in the change in state of a substance. An example is water when it is converted into steam or ice. He also described the specific heats of various substances. His work influenced his friend James Watt (1736–1819) who made critical advances in the design of the steam engine. This had been invented by Thomas Newcomen (1664–1729) but was inefficient. Watt’s modification greatly improved its performance and was a major factor in the development of the Industrial Revolution which had an enormous influence in history.
9.2 Brief Biography
Joseph Black was born in Bordeaux, France where his father was a wine merchant who himself had been born in Belfast but was of Scottish origin. Joseph’s mother was also from Scotland and it was she who taught her children to read English. At the age of 12 Joseph was sent to a private school in Belfast. There he was reported to have been an excellent scholar [7]. Four years later in 1744 he entered the University of Glasgow where he took the arts curriculum. However there is a note that in his fourth year he studied physics and was the favorite pupil of the professor of natural philosophy [7].
At the end of his arts course he studied medicine under Dr. William Cullen (1710–1790). This man was one of the most illustrious professors of medicine in the English-speaking world at the time and an important figure in the Scottish Enlightenment. When he moved to Edinburgh he was the physician of David Hume (1711–1776), the eminent philosopher, and had a wide circle of friends including Adam Smith (1723–1790), the economist. Cullen was a firm believer in the experimental method and he employed Joseph Black as his assistant in the laboratory who later reported that Cullen treated him “with the same confidence and friendship… as if I had been one of his own children” [13].
In 1752 Black moved to Edinburgh University to continue his medical studies. At the time, this institution had the finest medical education in the United Kingdom. It is not always appreciated that the Scottish universities at that time were far ahead of the better known English universities such as Oxford and Cambridge in the field of medicine.
Black was required to write a thesis for his M.D. degree in Edinburgh and he became interested in the properties of limewater which was thought to be valuable in the cure of kidney stone, a common ailment at the time. However it transpired that the action of limewater was a contentious subject between two of the major professors and Black therefore decided to work on a related topic, the properties of magnesia alba (magnesium carbonate). A feature of this research was Black’s use of accurate balances and in fact Black is credited with inventing the first accurate analytical balance. His experimental work on magnesia alba and his subsequent elucidation of the properties carbon dioxide are described below.
In 1756 Black returned to Glasgow where he was appointed lecturer, and later became professor. Remarkably, his research interests changed considerably. He became interested in the heat transfer that occurs particularly in a change of state, for example the transition from water to ice or water to steam. He had noticed that the change in state can occur over a prolonged period of time when a substance is heated or cooled without a change in temperature. For example snow at near freezing point gradually melts to form water over a period of time without a change in temperature. He introduced the term “latent heat” to refer to this phenomenon. He also discovered that different liquids have different capacities to take up heat, and he introduced the concept of “specific heat”.
James Watt (1736–1819), who was one of Scotland’s most famous engineers, came to Glasgow at the age of 18 and became an instrument maker to Black. The latter remarked “I soon had occasion to employ him to make some things… and found him to be a young man possessing most uncommon talents for mechanical knowledge and practice… which often surprised and delighted me in our frequent conversations together” [4]. Watt was influenced by Black’s work on latent heat and applied this knowledge to improve the steam engine. This had been invented by Thomas Newcomen (1663–1729) and was extensively used to pump water from mines but was very inefficient. The improvements developed by Watt played a critical role in the Industrial Revolution that made Britain a leader in industry.
In 1766 Black returned to Edinburgh where he concentrated on teaching. His lectures became famous and were read for many years. Later in life he had episodes of hemoptysis, presumably caused by pulmonary tuberculosis, and he died in Edinburgh in 1799. To many peoples’ surprise, his will indicated that he was quite wealthy.
For readers who want additional information, Ramsay wrote an early, readable biography of Black [13] and more extended accounts with corrections are by Guerlac [8, 9] and Donovan [7]. A collection of Back’s lectures was compiled by Robison [15] and an extensive series of letters between Black and Watt is available [14].
9.3 The Chemistry of Alkalis and Carbon Dioxide
The circumstances leading to Black’s work on alkaline chemicals were bizarre. Renal stones were apparently more common in the eighteenth century than they are now and they were a therapeutic challenge. “Cutting for stone”, that is operating to remove a renal stone or gravel from the bladder, was frequently described and in the period before anesthesia was a very painful and dangerous operation. As a result there was much interest in possible medical treatments. In 1739 a Mrs. Joanna Stephens invented a concoction that seemed to be helpful, and the English parliament voted her the sum of £5000 (an enormous amount in those days) for the recipe. This turned out to be a strange mishmash of eggshells, snails, soap and various other unlikely constituents, and as a result various medical people in the University of Edinburgh became interested in the properties of limewater which was assumed to play a role. A dispute developed between two professors, and although limewater interested Black, he thought it best to stay out of the controversy. He explained in a letter to his father “I found it proper to lay aside limewater which I had chosen for the subject of my Thesis. It was difficult and would have appeared presumptuous in me to have attempted settling some points about which two of the Professors themselves are disputing” [8]. He therefore chose to study a similar substance, magnesia alba (magnesium carbonate MgCO3) and for his MD thesis he wrote a disseration titled De humore acido a cibis orto, et magnesia alba (On the acid humour originating from food, and on magnesia alba). A year later he read a modified version of his dissertation as a paper titled “Experiments upon magnesia alba, quicklime, and some other alcaline substances” to the Philosophical Society of Edinburgh, and in 1756 this appeared in the second volume of the journal “Essays and Observations; Physical and Literary” of the Society [2] which later became the Royal Society of Edinburgh. The original paper is now very difficult to obtain but was reprinted by the Alembic Club in 1944 [3] (Fig. 9.2).
In early experiments Black added acid to magnesia alba and showed that it effervesced and lost weight. He used both distilled vinegar and oil of vitriol (sulfuric acid). In modern nomenclature the reaction was
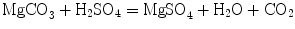
He also found that when magnesia alba was heated in a furnace, it also lost weight but the resulting material which he called magnesia usta did not lose weight when acids were added. Here the reaction was
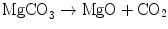
He realized that the action of heat on magnesia alba was the same as heating limestone which is calcium carbonate CaCO3. Again the reaction was
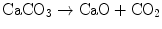
Black then looked at the properties of the “air” given off when magnesia alba was either treated with acid or heated. He found that it was not the same as atmospheric air. For example he reported in a letter to Cullen “I mixed together some chalk [CaCO3] and vitriolic acid… The strong effervescence produced an air or vapour, which, flowing out at the top of the glass, extinguished a candle that stood close to it; and a piece of burning paper immersed in it, was put out as effectually as if it had been dipped in water”. He also showed that it was toxic to animals that breathed it. For example sparrows “died in it in ten or eleven seconds” although “they would live in it for three or four minutes when the nostrils were shut by melted suet” [15] (page 231). He further found that when he bubbled his new gas through limewater it formed a white precipitate. This was calcium carbonate and the reaction was
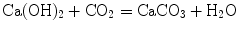
When Black used this test in a brewery he found that the same gas was given off in the process of alcoholic fermentation. He called the gas “fixed air” because in his experiments on alkalis the gas had been combined with a solid material. This was the first demonstration that gas was a weighable constituent of a solid body. As noted earlier, Black had developed very accurate chemical balances and these enabled him to show that when magnesium carbonate was heated and “fixed air” was liberated, there was a loss of weight. He also realized that it was the same gas as that described about 100 years earlier by Jan Baptist van Helmont who called it gas sylvestre and who produced it by adding acid to limestone. He also recalled that Stephen Hales (1677–1761) had suggested that the loss of weight of sal tartar (potassium carbonate) on heating was due to the loss of “elastic fluid” which he called fixed air [10]. Black also thought that it might be the same gas produced in the Grotto del Cano in Italy where it was known that people could survive if they were standing but dogs perished because the noxious gas being heavy remained close to the ground.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



