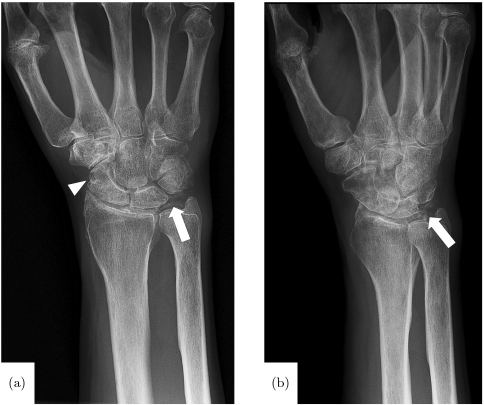
CHAPTER 10 Synovial joints contain a variety of different tissues, each type having varying degrees of vascularization. While some tissues are highly vascular, others, such as articular cartilage, are avascular, receiving nutrients via diffusion from synovial fluid or from the subchondral bone. The synovium consists of a lining (the layer closest to the synovial fluid) and a sublining, which is thicker and situated subjacent to the lining. Normal synovium is highly vascular, with a dense highly fenestrated capillary network, and the majority of the vessels being located within the portions of the sublining layer closest to the lining and capsule. In normal joints, vascular anatomy is typically highly organized; however, in the setting of arthritis and inflammation, vascular plasticity, the ability of the vascular system to remodel in response to stimuli, leads to abnormal redistribution of blood vessels, which may compromise both regulation of blood flow to the joint, as well as the functional capacity of the altered tissues.12 Non-synovial tissues comprising a typical synovial joint may include ligaments, articular cartilage, menisci, tendons, muscles, bursae, and bone. Tendons have been shown to have a variable vascular supply, with both vascular and avascular segments, as well as highly vascularized tendon sheaths which function similarly to the synovial lining, providing nutritional support to the superficial portions of the tendons. The fibrocartilaginous menisci are partially vascularized, with blood supply entering the meniscus peripherally, and diminishing toward the central and inner portions, such that the free edge of the meniscus is avascular. Devascularization progresses with age; vessels are only present in the portion of the meniscus closest to the joint capsule in adults over 50 years of age. The periarticular portions of bones are generally highly vascular, while the supporting joint capsule is typically avascular. Articular cartilage, as previously mentioned, is avascular and receives nutrient supply from the synovial fluid and subchondral bone.12 Vascular plasticity is a necessary adaptive mechanism to altered stresses and tissue damage incurred over the lifespan of a joint. While certain changes in the vascular anatomy may be considered a normal part of the aging process, vascular plasticity may also result in architectural changes that hinder normal tissue function. In patients with inflammatory arthritides, such as rheumatoid arthritis, as the synovial lining becomes inflamed and hyperplastic, the depth of blood vessels from the synovial surface increases, resulting in an increase in the distance over which nutrients must travel and therefore decreased efficacy with regard to nourishing the articular cartilage. Localized hypoxia may stimulate angiogenesis and a resultant immature vascular architecture. As this process progresses, the growing synovial pannus may invade nonsynovial structures such as adjacent bone and articular cartilage, resulting in local tissue damage. Additionally, vascularization of the normally avascular articular cartilage may result in functional compromise, leading to further joint damage.12 Inflammatory arthritis is an umbrella term encompassing a variety of arthritides, largely considered autoimmune, the hallmark of which is synovial inflammation, as opposed to osteoarthritis, which is considered primarily a degenerative process, though a component of inflammation may be present. Rheumatoid arthritis is a common and classic example of inflammatory arthropathy, and is classically described as a symmetric inflammatory peripheral polyarthropathy. Diagnostic criteria include polyarthritis involving three or more joints, positive serologic markers (these include rheumatoid factor, anti-cyclic citrullinated peptides, C reactive protein, and erythrocyte sedimentation rate), with duration of symptoms for more than six weeks, and exclusion of similar diseases, though not all of the diagnostic criteria are met in certain patients with rheumatoid arthritis. The arthropathy typically involves the hands and wrists, as well as a component of soft tissue swelling. Additional clinical features include morning stiffness and rheumatoid nodules. Early recognition and treatment of the disease is crucial in minimizing joint damage and resultant disability.35 A variety of other inflammatory arthropathies exist, and may be difficult to differentiate from rheumatoid arthritis, particularly early in the clinical course. More commonly encountered examples of these conditions include psoriatic arthritis, reactive arthritis (including Reiter’s syndrome), and arthritis of inflammatory bowel disease. Psoriatic arthritis is an inflammatory arthropathy encountered in patients with psoriasis, affecting approximately 4–30% of this patient population. Typically, joint involvement is polyarticular or oligoarticular, with various patterns of involvement described: •Distal, as characterized by involvement of the DIP joints •Asymmetric oligoarthritis, as characterized by asymmetric involvement of <5 small and/or large joints •Symmetric polyarthritis, which is often indistinguishable from rheumatoid arthritis •Arthritis mutilans, characterized by a destructive arthritis •Spondyloarthritis, characterized by sacroiliitis and/or spondylitis Additional clinical manifestations may include enthesitis, tenosynovitis, dactylitis, and nail lesions. Laboratory findings are non-specific, and may include elevated acute phase reactants and HLA-B27; rheumatoid factor is positive in a minority of patients.8, 14 Reactive arthritis is an uncommon disease affecting young adults, typically defined as a seronegative spondyloarthropathy resulting in arthritis following an infection; this term includes but is not limited to patients who fulfill the clinical criteria for Reiter’s syndrome. Organisms implicated in reactive arthritis include Chlamydia trachomatis, Yersinia, Salmonella, Shigella, Campylobacter, Escherichia coli, Clostridium difficile, and Chlamydophilapneumoniae. Clinical features include mono- or oligoarticular arthritis, often involving the lower extremities. The development of this arthritis often follows the initial infectious episode by several days to weeks. Additional signs and symptoms include enthesitis; dactylitis; conjunctivitis; oral, skin, and genital lesions; nail changes; and constitutional symptoms such as fever and malaise. Laboratory findings include evidence of preceding infection, elevated acute phase reactants, HLA-B27, and evidence of inflammatory synovitis. Treatment involves therapy directed at both the initial infection as well as the subsequent arthritis, which commonly involves non-steroidal anti-inflammatory drugs (NSAIDs) and/or glucocorticoids. The development of chronic refractory arthritis may require the use of disease modifying anti-rheumatic agents (DMARDs).17, 39 Arthritis associated with inflammatory bowel disease may occur in approximately 9–53% of this patient population, and appears to be more common in patients with ulcerative colitis and those Crohn’s patients with colonic involvement. Peripheral arthritis has been divided into type 1 (acute, pauciarticular, and remitting) and type II (chronic, polyarticular, and relapsing) subtypes. While type I most commonly involves the knees, type 2 most commonly involves the MCP joints. Additionally, sacroiliitis and spondylitis may occur. Laboratory findings are again somewhat nonspecific, and may include elevated acute phase reactants, HLA-B27, and evidence of inflammatory synovitis.32 Crystalline arthropathies result from the deposition of various crystals within the joints and periarticular soft tissues. Gout is the most common of these conditions, with an estimated prevalence of 3–8 million individuals in the United States, and involves the accumulation of monosodium urate crystals secondary to longstanding hyperuricemia. Clinical manifestations include recurrent attacks of inflammatory arthritis, chronic arthropathy, tophaceous deposits of urate crystals, nephrolithiasis, and chronic nephropathy. An acute attack of gout is characterized by severe pain, redness, and swelling typically involving the lower extremities, most commonly the great toe or knee. Inflammation, however, typically extends beyond the affected joint. Locations more commonly affected in recurrent gout include the ankle, wrist, fingers, and olecranon bursa. Definitive diagnosis of gout typically requires identification of characteristic urate crystals with the synovial fluid aspirated from an affected joint. In the absence of this finding, gout may be diagnosed tentatively based on a combination of classic clinical signs and symptoms in the setting of hyperuricemia and typical imaging findings.2, 5, 7 Various clinical syndromes have been associated with the deposition of calcium pyrophosphate (CPPD) within the tissues; these include: •Pseudogout: attacks of crystal-induced synovitis clinically similar to gout, but precipitated by CPPD. •Chondrocalcinosis: deposition of CPPD crystals within hyaline and fibro-cartilage, resulting in mineralization. •Pyrophosphate arthropathy: joint disease related to CPPD. The majority of joints affected by CPPD deposition are asymptomatic. In patients with acute pseudogout, the clinical manifestations closely resemble those of gout, with acute onset of pain and swelling, though most commonly involve the knee rather than the great toe. Differences in joint involvement, however, are insufficient to differentiate the two conditions — this is optimally accomplished by identification of characteristic crystals on microscopy of synovial fluid obtained from an affected joint. Longstanding joint disease related to CPPD may have a joint distribution resembling that of gout, rheumatoid arthritis, osteoarthritis, and/or neuropathic arthroses, complicating diagnosis.3, 30 Hemophilia is a term referring to bleeding disorders linked to deficiency of certain clotting factors, with hemophilia A (factor XIII deficiency) being more common than hemophilia B (factor IX deficiency). Most often, these patients tend to bleed into muscles, and, more commonly, joints, resulting in a characteristic arthrosis. Recurrent spontaneous hemarthroses in patients with severe disease commonly affect the ankles, knees, and elbows, contributing to synovitis and joint destruction related to iron deposition and fibrosis. Prophylactic treatment with factor replacement therapy has been shown to result in a decreased risk of spontaneous hemarthrosis and subsequently reduced risk of hemophiliac arthropathy.13 Pigmented villonodular synovitis (PVNS) is a rare condition of unclear etiology resulting in proliferation of synovial tissue within joints, tendon sheaths, and bursae. Diffuse and nodular forms are classically described, with the diffuse form being more common, and frequently affecting the larger joints. Clinical manifestations may include pain and swelling. Joint involvement is typically monoarticular. Extra-articular PVNS commonly involves the tendon sheath, resulting in a localized soft tissue mass, referred to as giant cell tumor of the tendon sheath.26, 36 Synovial chondromatosis is another rare proliferative disorder of the joints in which there is cartilaginous metaplasia of the synovium, which produces nodules of cartilage that subsequently break off and form numerous loose intra-articular bodies. These bodies, nourished by the synovial fluid, may grow, calcify, or ossify, ultimately resulting in mechanical damage to the articular cartilage. Clinical symptoms include pain, swelling, mechanical symptoms such as catching and locking, and limited range of motion. Joint involvement is typically monoarticular, most commonly involving the knee, followed by the hip, elbow, and shoulder. Patients with surgical implants may develop synovitis on the basis of a reaction to various types of wear debris. This most commonly occurs in the setting of joint arthroplasty, in which these implants are subject to load bearing and repetitive motion, predisposing the components to stresses leading to implant wear. Joint arthroplasties incorporating polyethylene components are extremely common; therefore, wear-induced synovitis related to polymeric debris is commonly encountered in patients with these implants. Particulate polymeric wear debris incite a histiocytic foreign body reaction in which tissue macrophages attempt to phagocytize particulate debris. For bodies too large to be phagocytized, macrophages fuse to form foreign body giant cells, secrete degradative agents, and encapsulate the body in a collagenous shell to isolate it from surrounding host tissues. Polymeric wear typically results in a synovitis and, if longstanding, can result in osteolysis about the implant.10, 11 Joint arthroplasties incorporating metal-on-metal bearings and junctions have been implicated in the development of a more aggressive type of wear-related reaction termed adverse local tissue reaction (ALTR). In this type of reaction, analogous to a type IV hypersensitivity reaction, wear debris acting as haptens complex with local proteins, are presented by surface complexes of local cells, and recognized by sensitized CD8 cytotoxic T cells, which destroy the “infected” presenting cell. CD4 helper T cells activate macrophages and produce interleukins which activate eosinophils. This process may result in a severe synovitis with aggressive and rapidly progressive tissue damage.10, 11 Hemarthrosis has been described as a complication of arthroplasty, and less commonly of arthroscopy. Recurrent bleeding into the joint space may lead to synovitis and joint destruction, in a similar fashion to hemophiliac arthropathy. Clinical manifestations include pain and swelling. Diagnosis commonly involves joint aspirate and/or imaging, in the setting of a history of preceding surgery.10, 23 While infection may occur in non-surgical settings, it is encountered with increased frequency in post-operative patients, in both acute/subacute and more chronic settings. In the more acute setting, postoperative edema and fluid may be present, complicating the diagnosis of infection.9, 10 A variety of imaging modalities have been employed in the diagnosis, monitoring, and post-treatment follow-up of pathologic conditions involving joint inflammation and synovitis. These modalities each have their particular strengths and weaknesses, and should be considered complimentary to a certain extent, though some modalities, such as magnetic resonance imaging (MRI), do offer a superior combination of sensitivity and specificity for diagnosis and monitoring of joint inflammation and synovitis. In a clinical context, diagnostic imaging continues to gain importance, not only in establishing an initial diagnosis, but also in terms of determining treatment strategy and efficacy. Conventional radiographs provide a general assessment of the osseous structures, with less sensitive evaluation of the soft tissues. While the synovium is not directly imaged utilizing radiography, this is often the first modality employed in the clinical assessment of suspected inflammatory conditions involving the joints. Imaging findings are therefore typically limited to the late effects of synovial expansion upon the osseous structures, such as erosions and areas of soft tissue mineralization (Fig. 10.1), though areas of soft tissue swelling and other soft tissue changes, such as tophaceous deposits in the setting of gout (Fig. 10.2), may also be evident. Ultimately, radiographs are relatively insensitive for detection of early disease, typically depicting only later manifestations of joint inflammation.19 However, radiographs, due to their ease of acquisition and ability to cover multiple joints, remain an effective modality for monitoring progression and/or treatment of known disease.19
JOINT INFLAMMATION AND SYNOVITIS
10.1 Introduction to Joint Inflammation and Synovitis
10.1.1 Joint tissues: Synovial
10.1.2 Joint tissues: Non-synovial
10.1.3 Vascular changes in joint inflammation
10.2 Joint Inflammation and Synovitis in Clinical Practice
10.2.1 Inflammatory arthropathies
10.2.2 Crystalline arthropathies
10.2.3 Hemophilia
10.2.4 Synovial proliferative disease
10.2.5 Post-surgical
10.3 Imaging of Joint Inflammation and Synovitis
10.3.1 Standard clinical imaging
10.3.1.1 Radio graphs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree