Ischemic Heart Disease and Complications of Myocardial Infarction
Michael Yeung
High-Yield Findings
Echocardiography is useful in the evaluation of acute chest pain by assessing possible wall motion abnormalities to identify areas of possible ischemia, as well as ruling out other acute causes of chest pain such as aortic dissection and pulmonary embolism.
Quick calculation of stroke volume (SV) may provide additional information in the unstable patient
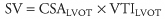
Color flow Doppler and agitated saline are helpful in the localization of ventricular septal rupture (VSR).
Presence of a pericardial effusion during or after a myocardial infarction (MI) is suspicious for left ventricular wall rupture.
The posteromedial papillary muscle is often affected in acute ischemic MR, given its single coronary supply from the posterior descending artery.
Assessment of the etiology and severity of acute MR may require TEE evaluation.
A neck diameter to maximal aneurysmal diameter ratio of <0.5 suggests pseudoaneurysm.
Evaluation of Chest Pain Syndrome in the Acute Setting
In the acute setting, the rapid detection of new wall motion abnormalities as well as mechanical conditions allows echocardiography to play an integral part in the evaluation of chest pain syndrome. In addition, the screening of other critical mediastinal entities such as aortic dissection, pulmonary embolism, and pericarditis makes echocardiography an essential diagnostic tool in the emergency room.
The previously discussed ischemic cascade (see Chapter 6) outlines the progression of reduced coronary flow and the ability of different diagnostic modalities to accurately detect its clinical manifestations. Echocardiography allows for the early detection of impaired myocardial relaxation even prior to the appearance of reduced systolic endocardial thickening.
Key Point:
The presence of ischemia can be detected by visualizing:
A lack of endocardial wall thickening (< 40%) during systole as well as appearance of new regional wall motion abnormalities.
A delay in the timing of endocardial thickening (usually completed within the first half of systole) leading to tardokinesis and post-systolic thickening.
Infarct can be differentiated from regions of ischemia by areas of akinetic, thinned-out myocardium.
Review of the ECG before obtaining the echocardiogram is important in order to localize and correlate the affected area. If the patient has a history of prior infarct, access to a prior echocardiogram is helpful for comparison of regional and overall LV function.
Contrast agents have greatly improved the echocardiographic diagnostic accuracy of MI in the acute setting by enhancing the detection of regional wall motion abnormalities as well as endocardial thickening.
For further discussion on coronary anatomy and regional distribution, please refer to Chapter 6.
Mechanical Complications of Acute Myocardial Infarction
Recurrent chest pain after MI should prompt suspicion and evaluation of recurrent ischemia, post-MI pericarditis, pericardial effusion, aortic dissection, or left ventricular rupture.
Cardiogenic shock after MI can be the result of right ventricular dysfunction, left ventricular dysfunction, acute MR due to papillary muscle rupture and tamponade due to LV rupture. Right-sided dysfunction can be differentiated clinically from left-sided dysfunction by the presence of marked neck vein distension, peripheral edema and the conspicuous absence of pulmonary edema.
A new holosystolic murmur after MI suggests either (1) ischemic mitral regurgitation due to papillary muscle dysfunction/rupture, (2) ischemic ventricular septal defect (VSD).
Left Ventricular Dysfunction and Cardiogenic Shock
Echocardiography provides important quantitative information regarding chamber size, ventricular systolic and diastolic function, valvular hemodynamics and pericardial involvement.
Stroke volume (SV) is an important indirect measurement that can assist in the evaluation of the patient’s hemodynamic status (CO = SV × HR).
SV can be reliably calculated by measuring the cross sectional area (CSALVOT) and velocity time integral (VTILVOT) of the left ventricular outflow tract by PW Doppler (SV = CSALVOT × VTI LVOT).
Mitral early blood flow (E) and annular (e′) velocities have been correlated with pulmonary capillary wedge pressure (PCWP). A septal E/e′ ≥15 corresponds to a PCWP >20 mmHg whereas an E/e′ ≤8 corresponds to a PCWP <15 mmHg. Intermediate E/e′ values of 9 to 14 are indeterminate for elevated PCWP and other factors should be evaluated (e.g., left atrial enlargement, presence of elevated pulmonary artery systolic pressure) (see Chapter 4).
Ventricular Septal DEFECT
VSD markedly increases risk of mortality and occurs within the first 3 to 6 days of MI.
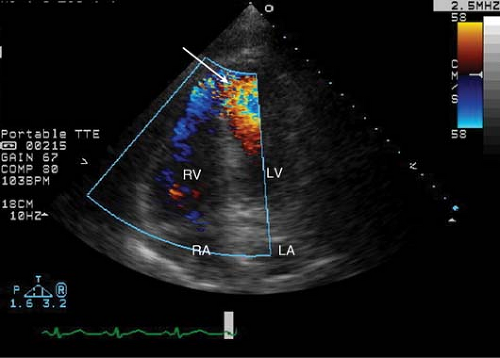
Figure 7-1. An apical four-chamber view with color Doppler showing a communication between the left and right ventricles in this apical VSD (arrow).
Risk factors include age (>65 years), hypertension, female gender, first infarction, and single-vessel coronary disease. Its classic presentation is recurrent chest pain and hypotension several days after MI along with the presence of a new harsh pan-systolic murmur and thrill in about 50% of the patients.
The left anterior descending and right coronary arteries are most commonly implicated in the development of VSD. Anatomically, the left anterior descending artery supplies the apical portion of the ventricular septum, while the right coronary artery gives rise to the posterior septal perforators that supply the basal inferoseptal wall (see Figs. 7-1 and 7-2).
VSDs may be simple or complex; with the latter often being associated with multiple areas as well as different dissection planes that track along the myocardium in a serpiginous fashion.
The use of color flow Doppler is essential to identify the location of the left-to-right shunt. Off-axis views may be needed to better localize the rupture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


