Ischemic Heart Disease
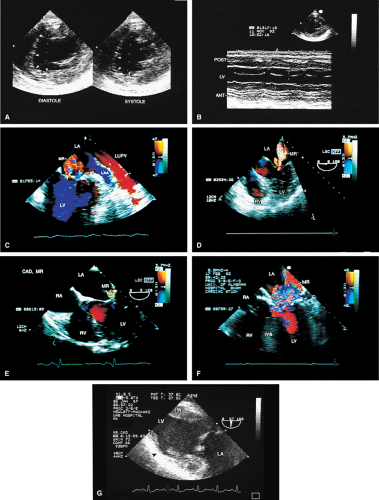
Careful transesophageal imaging can reveal a great deal of information about the anatomy of the coronary tree. The presence of stenosis is indicated by the compromise of the lumen. To be certain that what is being imaged is truly a stenosis rather than an artifact produced by an oblique section through the vessel, it is necessary to be able to see normal lumen on either side of the obstruction. Poststenotic dilatation may be present; it suggests a severe stenosis. Other markers of severe stenosis are an elevated pressure gradient across the stenosis by conventional Doppler and the presence of turbulence in the region of the stenosis on color-flow Doppler. The use of echocardiographic contrast may enhance flow visualization. Additional segments of the coronary vessels may be visualized and stenosis identified in these segments following contrast injection. The left main and proximal segments of the left anterior descending, left circumflex, and right coronary arteries, together with some of their branches, can be visualized by transesophageal echocardiography. With newer color Doppler equipment, smaller epicardial and intramyocardial vessels (e.g., septal perforators) can also be seen.
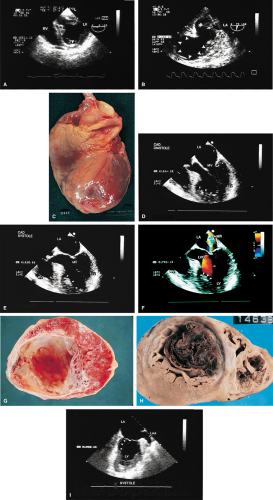
Wall motion abnormalities are also well visualized by transesophageal echocardiography, especially using the transgastric approach. Mitral regurgitation in ischemic heart disease can be recognized and its severity assessed. Mitral annular dilatation and dilatation of the left atrium and left ventricle imply long-standing, significant mitral regurgitation. When the head of the papillary muscle is ruptured, it can often be imaged prolapsing into the left atrium. In up to 30% of patients, however, the ruptured head may not prolapse into the left atrium. In these cases the diagnosis is made by observing the chaotic movement of the ruptured head in the left ventricle. Meticulous examination is important in these cases.
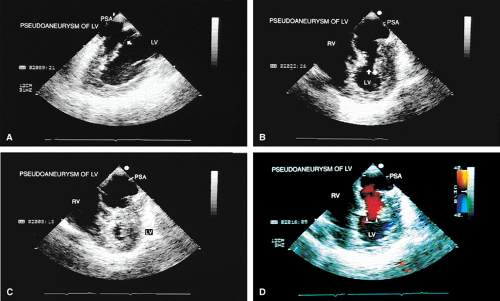
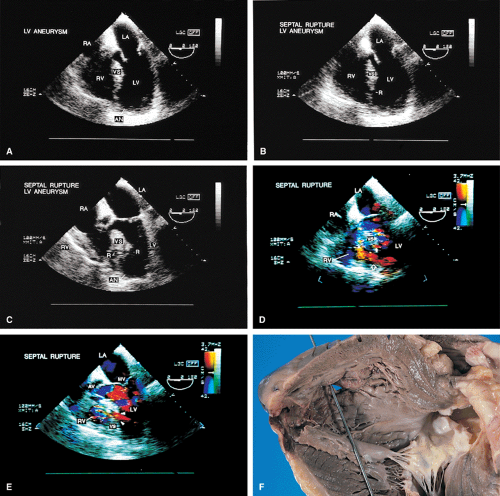
The term pseudoaneurysm refers to a rupture of the ventricle that is walled off by a clot before the patient experiences fatal tamponade. These lesions have a narrow neck (which distinguishes them from true aneurysms) that leads to a walled-off cavity. Pseudoaneurysms are inherently unstable in that they have a propensity to rupture. They should be resected even if discovered late after a myocardial infarction in an otherwise stable patient.
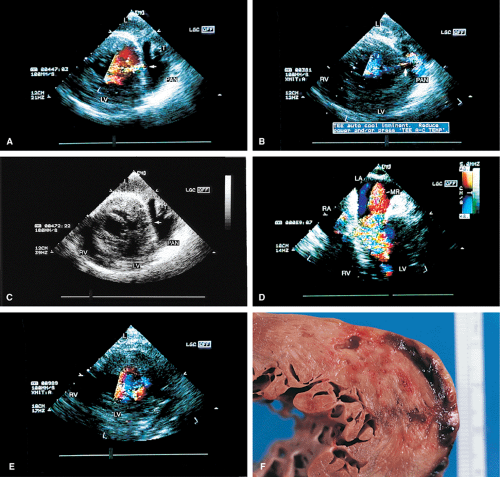
Other mechanical complications that can result from ischemic heart disease include ventricular septal rupture, which is easily seen on color-flow Doppler. Posterior ventricular septal defects are often best visualized using the transgastric approach.
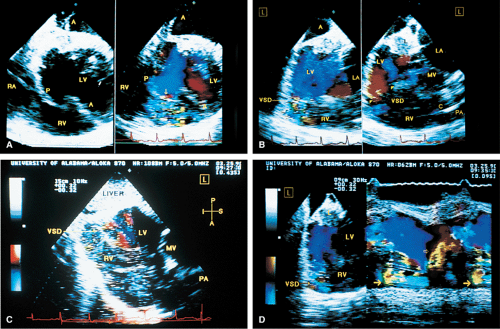 FIGURE 6.9. Ventricular septal rupture after acute inferior myocardial infarction. Transgastric views. A. Transverse plane imaging demonstrates mosaic color signals indicative of turbulent blood flow moving from the left ventricle (LV) through the posterior ventricular septum (P) into the right ventricle (RV). The arrow indicates a localized area of increased velocity with aliasing (flow acceleration) on the left ventricular side of the defect. Note that the defect is not identified on the non-color Doppler two-dimensional image. Top A
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|