Preferred strategy
Patient characteristics
Early Invasive
Recurrent angina or ischemia at rest or with low-level activities despite intensive medical therapy
Elevated cardiac biomarkers (TnT or TnI)
New ST-segment depression
Signs or symptoms of HF
New or worsening mitral regurgitation
High-risk findings from noninvasive testing
Hemodynamic instability
Sustained ventricular tachycardia
PCI within 6 months
Prior CABG
High-risk score (e.g., TIMI, GRACE)
Mild to moderate renal dysfunction
Diabetes mellitus
Reduced LV function (LVEF <40 %)
Conservative
Low-risk score (e.g., TIMI, GRACE)
Patient or physician preference in the absence of high-risk features
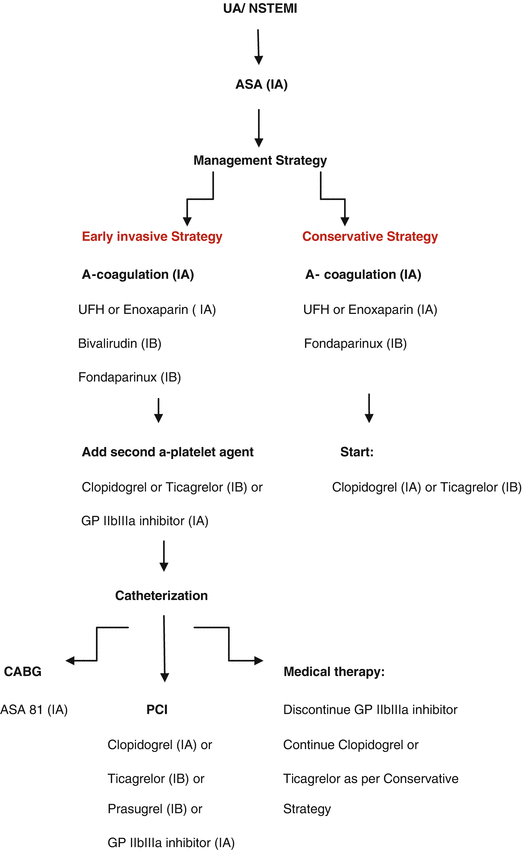
Fig. 6.1
Adapted from ACC/AHA guidelines for Initial Management of UA/NSTEMI (2012) (With permission from Jneid et al. [16])
STEMI (ST Elevation Myocardial Infarction)
STEMI comprises 25–40 % of ACS presentations in observational studies. According to the most recent National Registry of Myocardial Infarction 4 (NRMI-4), that number is around 29 % of ACS presentations [25, 26]. Significant improvements in the understanding the pathophysiology of atherosclerosis, especially the mechanism of acute myocardial infarction and intervening with appropriate therapies has resulted in not only a decreased incidence of recurrent STEMI, but also improved mortality rates, both in-hospital (5–6 %) and at 1-year (7–18 %) [27, 28].
Approximately 20–30 % of patients presenting with STEMI are women [29, 30]. In earlier STEMI trials women were significantly underrepresented. This is thought to be due to the basic pathophysiologic difference in ACS presentation between men and women. Women presented more often with NSTEMI as they tended to have more atherosclerotic plaque erosion whereas men had plaque rupture and vessel occlusion [8, 31]. However the proportion of women, especially younger women, has increased in the STEMI population. This proportionate increase in women seems to be related to an increase in risk factors such as smoking and obesity in these studies suggesting possible change in plaque pathophysiology and, therefore, plaque instability. Also, women tend to have increased mortality after a STEMI compared to men despite receiving optimal or similar therapy to men [29, 32]. Women also seem to have higher incidence of complications from the therapy administered including more bleeding from antiplatelet and lytic therapy, more vascular complications, and also increased rates of renal failure [32–35]. Thus the increase in the incidence of STEMI in women combined with the higher mortality despite optimal therapy, and higher complication rates with invasive and non-invasive therapy make them a unique group requiring special consideration and more dedicated research.
The treatment of STEMI includes the acute phase and long term management phase. The acute management includes pre-hospital management, reperfusion strategies, and the management of the hospitalization including the complications of the MI and the reperfusion strategy selected.
Pre-hospital Management
Initial studies raised the concern, which was probably justified during that time period that women were not getting standard of care management for their STEMI presentations. In the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines) registry female gender was a strong independent predictor of a failure to receive reperfusion therapy among patients who had no contraindications [36]. Women included in the NCDR (National Cardiovascular Data Registry) ACTION Registry–GWTG (Get With The Guidelines) presented later after symptom onset, had longer door-to-fibrinolysis or door-to-balloon (or device) (D2B) times, and did not receive aspirin or beta blockers within 24 h of presentation as often [36]. However, a recent assessment of the effects of a statewide program for treatment of STEMI suggests significant improvements in treatment times that were similar for whites and blacks and for women and men [37]. Recognizing the problem has been the real challenge in pre-hospital management of STEMI in women. Women tend to present with atypical symptoms delaying the initial diagnosis [38]. However, once the diagnosis has been established the morbidity and mortality are driven by the patient’s co-morbidities rather than by sub-optimal treatment. There are still regional differences in symptom recognition to ECG time and also symptom onset to hospital time as demonstrated by multiple studies [39, 40]. Thus, there is a need for more campaigns to educate women and health care workers to recognize atypical symptoms for angina, and obtain a timely ECG as soon as it is recognized.
Once EMS suspects a possible ACS on a call the patient is asked to chew 324 mg (four 81 mg tablets) of aspirin to help hasten the onset of systemic action. Nitroglycerin in the appropriate clinical setting may help with some symptom relief, but has not demonstrated any mortality benefit in trials. Also, caution should be exercised with inferior STEMI patients as right ventricular involvement of the infarction could potentially cause significant hypotension with nitroglycerin. Oxygen therapy is routinely administered and has no proven harm and opioids seem to relieve anxiety and pain. Activation of the STEMI alarm at hospitals that perform primary PCI by the EMS personnel from the field has resulted in shorter door to balloon times resulting in better outcomes [41].
ED and Hospital Management
Once in the emergency room, the focus shifts to choosing the appropriate reperfusion strategy [42]. If primary PCI is available, it should be performed in the shortest time possible. The ACC/AHA guidelines recommend that reperfusion (the door to balloon time) be achieved in <60 min from time of arrival to the ED. If primary PCI is not available and the nearest primary PCI center is >120 min away, then the ACC/AHA Class 1 recommendation is to consider thrombolytic therapy if no contraindications exist. If thrombolytic therapy is the reperfusion strategy of choice for the patient, it should be accomplished within 30 min of arrival to the ED. Dual anti-platelet therapy should be initiated as soon as possible. Currently, there are three drug options are available for patient’s undergoing urgent PCI: clopidogrel, prasugrel, and ticagrelor. No published literature exists on gender specific differences in the type of thrombolytic therapy or anti-platelet agents.
PCI: Invasive reperfusion therapy has no significant gender related issues per se. In general, women tend to have more diffuse disease in significantly smaller vessels making them less than ideal for PCI. Women tend to be older than men at the time of presentation, with more comorbidities and, therefore, they tend to have more complex lesions. For these reasons the procedural success rate in women in various studies is lower than that of men [42]. Also, patients with failed PCI attempts have a higher short term and long term mortality, in some studies up to 20 and 50 %, respectively [43]. The evidence suggests that MACE is reduced with placement of a stent when compared to balloon angioplasty alone in STEMI. Even though they have smaller caliber vessels and a higher incidence of DM requiring smaller stents, both risk factors for in-stent restenosis, women tend to have a lower incidence of restenosis compared to men [44]. The drug eluting stents seem to perform equally well in women and men [45]. Thus, it appears that choice of stent should not be guided by gender differences.
Early Invasive Therapy: Adjunctive Pharmacotherapy
Antiplatelet Therapy
Aspirin
Acetylic Acid (ASA) irreversibly blocks cyclo-oxygenase and the synthesis of arachadonic acid- derived thromboxane A2- a strong promoter of platelet aggregation. ASA significantly inhibits platelet function within 60 min of administration [46]. ASA therapy reduces cardiovascular mortality, which is well established. ASA (four 81 mg tablets) should be administered as soon as possible after presentation with acute coronary syndrome and continued indefinitely [16]. The ISIS-2 trial (International Study of Infarct Survival- 2) demonstrated that reduction in mortality with ASA vs. placebo in acute MI was lower in women (16 %) when compared to men (22 %), although the difference was not statistically significant [47]. Further analyses revealed no clear outcome benefit of ASA in doses greater than 100 mg/day compared with lower doses in women, despite previously reported higher platelet reactivity, and resulted in higher incidence of bleeding [48–51].
P2Y12 Receptor Inhibitors
Thienopyridines: Clopidogrel and prasugrel, selectively and irreversibly inhibit the P2Y12 ADP platelet receptor, which plays a critical role in platelet activation and aggregation. Both agents work synergistically with ASA in providing greater platelet inhibition than either agent alone [46].
The prodrug, clopidogrel, is inactive in vitro, and is metabolized by a cytochrome P450-3A4 to become the active metabolite. The standard 300 mg loading dose of clopidogrel requires up to 6 h for maximal antiplatelet effect, whereas a 600 mg dose achieves that goal in about 2 h. Full platelet inhibition is achieved within 2–3 days of therapy onset with a daily dose of clopidogrel 75 mg following a loading dose. Platelet function recovers in 5–7 days after discontinuation of the drug [46]. The pivotal trial for Clopidogrel, the CURE trial (Clopidogrel in Unstable angina to prevent Recurrent Events), evaluated 12,562 patients with UA/NSTEMI who were randomized to clopidogrel or placebo, and demonstrated a 20 % relative risk reduction in the composite primary end point of death, MI, stroke at 1 year, at the cost of 1 % increased risk of major bleeding. Similar benefits were observed in women (RR 0.77, 95 % CI 0.52–1.15) and men (RR 0.65, 95 % CI 0.48–0.87) [49, 52]. A significant limitation of the trial was the non- routine use of an early invasive strategy at the time of study as only 44 % of patients underwent cardiac catheterization at the time of index hospitalization. A 9.6 % rate of major bleeding was observed among patients who received clopidogrel within 5 days of CABG [49, 52]. Accordingly the ACC/AHA guidelines recommend that clopidogrel should be discontinued 5–7 days before CABG unless the urgency of CABG outweighs the risk of bleeding (class l indication) [16, 42].
Prasugrel is a novel thienopyridine and prodrug and, like clopidogrel, requires conversion to an active metabolite before binding to the platelet P2Y12 receptor to confer antiplatelet activity. Prasugrel inhibits ADP–induced platelet aggregation more rapidly, more consistently, and to a greater extent than does clopidogrel in patients with coronary artery disease, undergoing PCI [53]. The TRITON- TIMI 38 trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel- Thrombolysis in Myocardial Infarction 38) was a landmark trial that studied prasugrel (60 mg loading dose, followed by 10 mg/day) in patients with ACS (74 % with UA/NSTEMI) compared to standard dose Clopidogrel (300 mg followed by 75 mg/day) in 13,600 patients [53]. The prasugrel loading dose was administered before, during or within 1 h after PCI but only after coronary anatomy had been defined. Prasugrel was associated with 2.2 % absolute risk reduction and a 19 % relative risk reduction with respect to the primary end point (a composite of cardiovascular death, MI, stroke). Data from a landmark analysis demonstrated that prasugrel was superior to clopidogrel within hours of administration, which is consistent with its more rapid onset of action. The rates of stent thrombosis were significantly reduced when compared to clopidogrel, from 2.4 to 1.1 % (p < 0.001) by prasugrel. However, prasugrel was associated with significant increase in the rate of bleeding complications (2.4 % for prasugrel compared to 1.8 % for clopidogrel, HR 1.32, 95 % CI1.03–1.68, p = 0.03), including the rates of fatal bleeding, and CABG- related bleeding [53].
A post hoc analysis suggested three groups of ACS patients who did not have net clinical benefit (defined as the rate of death, MI, stroke or non- CABG related bleeding) from prasugrel. Patients with history of stroke or TIA had net harm from prasugrel therapy and patients age ≥75 years or with a body weight of <60 kg had no net benefit from prasugrel therapy. In both treatment groups patients with at least one of these risk factors had higher rates of bleeding than those without them [53]. While there are not gender specific data available for prasugrel, female patients tend to be older and generally weigh less, often falling into one of the relative contraindication categories.
Ticagrelor is a novel non-thienopyridine, reversible P2Y12 antagonist, which does not require metabolic activation in vivo, has a rapid onset of action, greater inhibition of platelet aggregation and significantly faster reduction in effect at drug termination when compared to clopidogrel. The pivotal trial for ticagrelor, the PLATO trial (Study of Platelet Inhibition and Patient Outcomes) enrolled 18,624 patients with ACS (16.7 % with UA, 42.7 % with NSTEMI) and randomized them to receive a standard dosing regimen of clopidogrel (300 mg loading dose followed by 75 mg/day) or ticagrelor (180 mg loading dose, followed by 90 mg twice daily) [54]. The composite endpoint of cardiovascular death, MI or stroke as well as the primary safety end point of major bleeding event was compared between the groups. Ticagrelor was associated with 2 % ARR (HR 0.84, 95 % CI 0.770.92, p = 0.0003) of the primary endpoint in patients with NSTEMI. The benefits of ticagrelor were present at 30 days of therapy and persisted for up to 360 days, and were similar across invasive or conservative strategies. Notably Ticagrelor was associated with 1.4 % ARR in all- cause mortality and lower rates of definite stent thrombosis without an increase in the risk of bleeding, including CABG- related bleeding [54].
The pivotal trials for prasugrel and ticagrelor demonstrated relatively smaller reductions in the primary endpoint in women compared to men, however, neither of the studies clearly defined a significant interaction between gender and treatment assignment nor were they powered to analyze treatment interactions between subgroups [55].
GP IIb/IIIa Inhibitors
Abciximab, tirofiban, eptifibatide block the platelet GP IIb/IIIa receptor mediated final common pathway of platelet aggregation by preventing binding of fibrinogen [46]. Their efficacy has been well established during PCI procedures in patients with UA/NSTEMI, particularly among high risk patients, such as those with elevated cardiac biomarkers and diabetes mellitus [56, 57].
Although treatment with GP IIb/IIIa antagonists was associated with a significant reduction in death or MI at 30 days in men, women had worse outcomes with such therapy [55, 56]. This difference can potentially be explained in part by clinical characteristics of the patients. Women enrolled in those trials tended to be older, had more extensive MI’s and had more co-morbid conditions. When risk was further stratified by troponin level no gender differences were seen [55]. More recent trials have not demonstrated significant differences in outcomes stratified by gender, potentially due to concomitant use of clopidogrel [55, 58].
Anticoagulant Therapy
Unfractionated Heparin (UFH) is an indirect thrombin inhibitor, which complexes with antithrombin (AT), converting this circulating cofactor from a slow to rapid inactivator of thrombin, factor Xa, and to a lesser extent, factors XIIa, XIa and IXa [46]. The anticoagulant response to a standard dose of UFH varies widely among patients, which makes it necessary to monitor the response in each patient, using the activated clotting time (ACT) and to titrate the dose to the individual patient anticoagulation level. In women UFH has been commonly used in combination with ASA for medical treatment of UA/NSTEMI as well as in early invasive strategy with PCI [15].
A weight adjusted dosing regimen is standard for all patients with a goal ACT: 250–350 s [15]. Lower doses of heparin and therefore lower ACTs should be considered in women and elderly patients, especially when used in combination with GP IIb/IIIa inhibitors during PCI [59]. It has also been recognized that prolonged therapy with UHF has been associated with increased risk of bleeding without adding benefit and therefore, no additional heparin is recommended after completion of the PCI procedure [59].
Low Molecular Weight Heparin (LMWH)
Enoxaparin and Dalteparin are low molecular weight heparins, which are fragments of UFH produced by a controlled enzymatic depolymerization process. Similar to unfractionated heparin they work by binding to antithrombin 3, causing a conformational change that accelerates the interaction of AT with thrombin and factor Xa [46]. Compared to UFH, LMWH’s anti- Xa activity predominates over its anti-thrombin activity. In addition it has better bioavailability, is more conveniently administered by subcutaneous injections, produces more predictable dose response, causes less platelet activation and results in less heparin induced thrombocytopenia (HIT) [59]. The efficacy and safety of the a commonly used LMWH, enoxaparin, was studied in patients with UA/NSTEMI undergoing PCI in 2 trials. The A-Z Trial (Aggrastat to Zocor phases) enrolled 3,987 patients, 29 % of which were women, and the SYNERGY trial (Superior Yield of the New Strategy of Enoxaparin, Revascularization, and Glycoprotein IIb/IIIa Inhibitors) enrolled 9,978 patients of which 34 % were women. Both studies met their non- inferiority endpoints, demonstrating no significant reduction in benefit of enoxaparin when compared to UFH in either women or men undergoing PCI for ACS. No significant difference in the rate of major bleeding was found between two agents [60, 61].
Direct Thrombin Inhibitors
Bivalirudin is a synthetic direct thrombin inhibitor with a short half-life. It acts on both circulating and clot-bound thrombin, with much higher thrombin specificity when compared to UFH and no platelet activation effect. It has been extensively studied in patients with ACS undergoing PCI. In a meta-analysis of 8,497 patients undergoing elective or urgent PCI for ACS, comparing direct thrombin inhibitors to UFH, there was a significant reduction in death or MI (4.6 % vs 6.6 %, RRR 0.68, 95 % CI 0.57–0.83, p < 0.001) and lower rates of bleeding complications [62]. In the REPLACE 2 trial (Randomized Evaluation in Percutaneous Coronary Intervention Linking Angiomax to Reduced Clinical Events) bivalirudin with provisional or bailout use of GB IIb/IIIa inhibitor was compared to planned use of UFH with GP IIb/IIIa inhibitors in 6,010 patients (26 % women) undergoing PCI looking at ischemic and bleeding outcomes. In that trial female gender was an independent predictor of death and bleeding complications [63]. Among women treated with bivalirudin or heparin there was no difference in the individual or composite ischemic end points, but bivalirudin was associated with a significant reduction in major bleeding at 30 days that persisted out to 6 months (34.1 % with UFH vs 19.7 % with bivalirudin, p < 0.001) [63].
The landmark ACUITY trial confirmed the reduced bleeding event rates with Bivalirudin and its non- inferiority with respect to ischemic outcomes in patients with UA/NSTEMI undergoing PCI [64]. In this trial 13,819 patients (4,157 were women, 30.1 %) were enrolled, and randomized into three different treatment arms: heparin (UFH or enoxaparin) +/− GPI, or bivalirudin plus GPI, or bivalirudin alone. Major bleeding (not related to CABG), composite ischemia (death, MI, revascularization), and net clinical outcome (composite ischemia or bleeding) were compared. Women had similar 30 days composite ischemic event rates as men (7 %vs 8 %, p = 0.07), but greater 30 day rates of major bleeding (8 % vs 3 %, p < 0.001) and net clinical outcomes (13 % vs 10 %, p < 0.001). One year ischemic outcomes and mortality were similar in both genders [64].
At 30 days women treated with bivalirudin as compared to GPI + UFH had significantly less major bleeding events ( 5 % vs 10 %, p < 0.001) and the same rate of composite ischemia ( 7 % vs 6 %, p = 0.15). There was no difference found in 1 year composite ischemia and mortality endpoints in women with respect to treatment with bivalirudin vs UFH + GPI. In summary, bivalirudin monotherapy compared to GPI- based strategy in women resulted in significantly reduced bleeding rates, but similar 1 year composite ischemia and mortality rates [64, 65].
The medical management of acute coronary syndromes is outlined in the AHA/ACC guidelines as noted in Table 6.2.
Table 6.2
Summary of ACC/AHA guidelines for the management of Patients with Unstable Angina/N-ST Elevation Myocardial Infarction (2007, 2011, 2012)
Anti- ischemic therapy |
Class I indications: |
Bedrest and Telemetry |
Oxygen (maintain saturation >90 %) |
Nitrates (sl nitroglycerin x3, oral/topical, iv for ongoing ischemia, heart failure, hypertension) |
Oral Beta Blockers: should be initiated in first 24 h if no contraindications exist |
ACE inhibitors: should be initiated in first 24 h for heart failure or EF <40 % |
ARB: for patients who cannot tolerate ACE inhibitors |
Statin |
Class IIa indications: |
Iv Beta Blockers if no contraindications exist ( see below) |
ACE inhibitors in all patients with ACS |
Class III (what should not be given) |
Nitrates if BP <90 mmHg or RV infarction |
Nitrates within 24 h of Sildenafil or 48 h of Tadalafil use |
Immediate release dihydropyridine CCB in the absence of Beta Blocker therapy |
Iv ACE inhibitors |
Iv Beta Blockers in patients with: acute heart failure, low output state or cardiogenic shock, PR interval >0.24 s, 2nd or 3rd degree heart block, active asthma, or reactive airway disease |
NSAID’s and Cox-2 inhibitors |
Anti-platelet therapy |
Class I indications |
ASA (162–325 mg), non-enteric coateda, b |
Clopidogrel for patients with ASA allergy/intolerance (300–600 mg loading dose followed by 75 mg maintance dose) ot Ticagrelor |
GI prophylaxis should be given to patients with history of GI bleeding |
The use of dual a-platelet therapy or GP IIb/IIIa should be evaluated based on whether and invasive or conservative strategy is used |
Patients with UA/NSTEMI in whom invasive strategy is selected should receive dual a- platelet therapy on presentation ( Class1A); in addition to ASA, choice of 2nd a- platelet agent should include one of the following: |
Before PCI: |
Clopidogrel ( loading dose 300–600 mg, followed by 75 mg daily for minimum 12 months) or |
Ticagrelor or |
An iv GP IIb/IIIa inhibitor, preferably iv eptifibatide or tirofiban |
At the time of PCI: |
Clopidogrel if not started before PCI (600 mg should be given as early as possible before or at the time of PCI, or |
Prasugrel (60 mg loading dose should be given promptly and no later than 1 h of PCI, followed by 10 mg daily for minimum 12 months) or |
Ticagrelor (180 mg loading dose, followed by 90 mg twice daily for minimum 12 months) or |
Iv GP IIb/IIIa inhibitor |
Anticoagulant therapy: relative choice depends on invasive vs conservative strategy |
Class I indications |
Unfractionated Heparin or |
Enoxaparin or |
Bivalirudin or |
Fondaparinux |
Outcomes by Device
Bare Metal Stents and Drug Eluting Stents
In clinical trials, the outcomes with bare metal stenting in women are similar to men in regards to in- hospital mortality and target lesion revascularization [66]. In a real world experience of patient and lesion subsets, mortality rates are similar or higher in women after stenting because of other confounding risk factors rather than female gender [63]. An analysis by Onuma, et al. which compared bare metal stents (BMS) with drug eluting stents (DES) in women showed no difference in 3- year outcomes between the genders, despite worse baseline clinical characteristics in women [67]. In patients treated throughout the BMS and DES eras, there were no differences by gender in all- cause mortality, MI or target lesion revascularization (TLR) at 3 years. Higher procedural complexity was observed in the DES era. At 3 years MACE and revascularization rates in women were significantly lower with DES than BMS (HR for TLR 0.52, 95 % CI 0.36–0.75, HR for MACE 0.63, 95 % CI 0.48–0.83) [64]. Another study by Fath- Ordoubadi et al., demonstrated equal efficacy and safety of DES in both genders [68]. Multivariate analysis in this study, which evaluated age, hypertension, diabetes mellitus, and number of diseased vessels, demonstrated that gender was not a predictor for adverse outcomes [68].
Gender-based differences in long-term clinical and angiographic outcomes after coronary revascularization with DES were also analyzed in a recent study by Stefanini, et al. [69]. After adjustment for baseline differences, women and men had a similar risk of cardiac death or MI (odds ratio [OR]: 1.13, 95 % confidence interval [CI]: 0.82–1.56, p = 0.44), cardiac death (OR: 1.04, 95 % CI: 0.61–1.80, p = 0.87), and MI (OR: 1.07, 95 % CI: 0.75–1.53, p = 0.71) at 2 years. Similarly, the risk of target lesion revascularization (OR: 1.09, 95 % CI: 0.77–1.54, p = 0.62), target vessel revascularization (OR: 0.88, 95 % CI: 0.63–1.22, p = 0.43), and definite or probable stent thrombosis (OR: 0.73, 95 % CI: 0.38–1.38, p = 0.33) were comparable for women and men. Follow-up angiography demonstrated no differences in terms of in-stent late loss (0.18 ± 0.54 mm vs. 0.20 ± 0.99 mm, p = 0.76) and in-segment binary restenosis (8.5 % vs. 8.5 %, p = 0.76) [69].
Female Gender and Adverse Outcomes After PCI
Many discrepancies have been reported regarding the prognosis and differences in adverse outcomes in women compared to men undergoing PCI. Registries and randomized trials analyzing early and late major adverse cardiovascular ischemic events have reported increased [70–72], neutral [73–75] and, even lower risk [76, 77] outcomes for women as compared to men. It has been suggested that women with ACS at younger age had higher mortality compared with men at the same age [4, 6, 78, 79]. After adjustment for several risk factors female gender remained a risk factor for in-hospital mortality in ACS for patients aged 51–60 [80]. An analysis by Duvernoy et al., demonstrated that women had a higher frequency of adverse outcomes after PCI, including in- hospital death, vascular complications, stroke, and MACE after adjusting for clinical and procedural characteristics. The relationship of increased risk of death and MACE was no longer present after adjusting for renal function and lower body surface area [81], suggesting an important impact of impaired kidney function and smaller size of female patients on long- term prognosis. Another analysis by Pendyala, et al., revealed a significant difference between the genders in independent correlates of mortality and MACE, and different impact of traditional prognostic correlates in women and men [82]. Women tend to have similar or lower rates of restenosis when compared to men despite smaller vessel sizes and higher prevalence of DM [71, 83, 84], factors usually associated with higher restenosis and revascularization rates. The reasons for these findings are unclear.
Bleeding Risk and Vascular Complications
Data from several sources suggest that gender-related differences exist in bleeding complications in ACS patients. In a multivariate analysis by Subherval et al., female gender was identified as an independent predictor of major in-hospital bleeding (OR 1.31, 95 % CI 1.23–1.39) in patients with ACS [83]. In the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA guidelines) registry, women who underwent PCI for UA/NSTEMI, had a much higher risk of major in-hospital bleeding compared to men (14.1 % vs 5.9 %, p < 0.001) [84].
Women undergoing PCI tend to have more access- related bleeding. In contrast, men tend to have GI bleeding after an MI. Possible explanations for this finding include smaller blood vessels in women and specific vascular reactivity [55]. In the same registry, a significant interaction was found between gender, GP IIb/IIIa inhibitor use and bleeding risk (p = 0.014). The increased risk of bleeding due to use of GPIIb/IIIa or anticoagulant agents like UFH or enoxaparin, may be related to excessive dosing of those agents (15 % of major bleeding events observed were due to excess drug doses) [64, 83, 84]. After adjusting for age, weight, and renal function, women remained at higher risk for excessive dosing and major bleeding events, as the authors found that excessive dosing of antithrombotic agents occurred in 72 % of women compared to 27 % of men [85, 86]. However, the ISAR- REACT and ACUITY trials [85, 86] provide data to suggest that bleeding complications remain higher among women compared to men even when they receive appropriate doses of antithrombotic agents.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


