Major complications
Total
<1.0 %
Death
None
Tamponade with pericardiocentesis
0.08–0.80 %
Hemothorax or pneumothorax
None
Permanent complete AV block with permanent pacemaker needed
<0.04 %
Urgent cardiac surgery
None
Minor Complications
Total
<5.0 %
Small pericardial effusion
0.7–1.8 %
Conduction abnormalities not requiring permanent pacemaker
0.2–3.7 %
Arrhythmia
0.2–1.1 %
Tricuspid regurgitation
0.43 %
AHA/ACCF/ESC Joint Statement
The joint scientific statement released by the American Heart Association, American College of Cardiology Foundation, and the European Society of Cardiology in 2007 outlines the role of endomyocardial biopsy in the diagnosis and treatment of cardiovascular disease. The statement identifies specific clinical scenarios in which endomyocardial biopsy is recommended because “specific myocardial disorders that have unique prognoses and treatment are seldom diagnosed by noninvasive testing” [8, 9]. Clinical scenario three directly relates to cardiac sarcoidosis. Although the statement acknowledges that the diagnostic rate for cardiac sarcoidosis is low, they recommend endomyocardial biopsy for patients who present with unexplained heart failure of greater than 3 months’ duration, with a dilated left ventricle, and new ventricular arrhythmias, second-, or third-degree heart block, or failure to respond to usual care within 1–2 weeks [10]. The recommendation emphasizes the important distinction between giant cell myocarditis and cardiac sarcoidosis because early transplant is recommended in the former, and corticosteroids and possibly implantable cardiac defibrillator in the latter. In addition, despite high rates of heart block, heart failure, and arrhythmias, survival is better in patients with cardiac sarcoidosis than with idiopathic giant cell myocarditis [11] (Table 8.2).
Table 8.2
Clinical scenarios where endomyocardial biopsy for cardiac sarcoidosis can be considered
Heart failure for more than 2 weeks with dilated left ventricle and new ventricular arrhythmias, second-or third-degree heart block, or failure to respond to usual care within 1–2 weeks: scenario suggests possible cardiac sarcoidosis versus giant cell myocarditis versus idiopathic granulomatous myocarditis, where diagnosis has therapeutic implications |
Suspected arrhythmogenic right ventricular cardiomyopathy (ARVC): multiple case reports of cardiac sardoiosis mimicking ARVC |
Young patient with unexplained second- or third-degree heart block |
Unexplained ventricular arrhythmias |
Strong suspicion of cardiac sarcoidosis with cardiac symptoms and equivocal non-invasive testing |
Patients with diagnosis of extracardiac sarcoidosis with suspected cardiac sarcoidosis in whom diagnosis confirmation will substantially change management |
Image-Guided Biopsy
Imaging-guided biopsies may improve the diagnostic yield and overcome the sampling error that limits the sensitivity of conventional endomyocardial biopsy. 18FDG-PET scans and cardiac MRI with delayed gadolinium-enhancement can detect areas of active inflammation and/or damage. Using these scans to direct endomyocardial sampling towards areas of tissue that may be more likely to demonstrate histopathologic evidence of sarcoidosis has been demonstrated to be effective in isolated case reports. A case in which conventional biopsy of the right ventricular septum failed to yield a diagnosis, but a repeat MRI-guided biopsy of areas of transmural delayed enhancement demonstrated diagnostic non-caseating granulomas was published by Borchert et al. [12]. A paper by Kandolin et al. describes their experience in changing diagnostic strategy in detecting isolated cardiac sarcoidosis without clinically apparent extracardiac sarcoidosis. Their experience suggests an improvement in detection rates with repeated and imaging-guided biopsies of cardiac and mediastinal lymph nodes [13].
Electroanatomical Mapping-Guided Biopsy
Three dimensional electroanatomical mapping (EAM) systems have proven to be invaluable tools as part of a strategy for mapping and ablating complex arrhythmias. In addition, these systems have also been shown to demonstrate high fidelity reconstruction of chamber dimension and quantification of viable myocardium [12]. Electrically inert tissue that results from the scar left behind after myocardial infarction or with infiltrative cardiomyopathy will be reflected by a reduced voltage recorded by a roving catheter in the endocardium or epicardial space [14]. As shown in Fig. 8.1, an area of reduced voltage recorded with EAM corresponds to myocardial scar and granulomatous infiltration as identified in histopathologic studies. The presence of mid-myocardial scar is more difficult to accurately record with a bipolar catheter; however, unipolar mapping may identify this possibility as well [15].
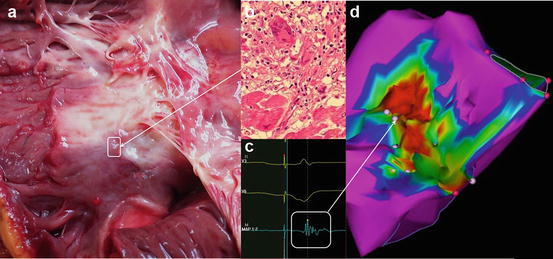

Fig. 8.1
Gross examination of an explanted heart at autopsy in a patient with cardiac sarcoidosis, revealing granulomatous myocardial scarring of the RV septum (a). (b) Shows the characteristic noncaseating granuloma infiltrating the myocardium at the border-zone with hematoxylin-eosin stained tissue at 400× power. (c) Shows an intracardiac electrogram recorded at a similar border-zone site. (d) Is the electroanatomical map of the right ventricular septum of this same patient recorded 2 years prior to autopsy. This area of reduced voltage with heterogeneous distribution of low amplitude electrograms corresponds to the myocardial scar caused by sarcoidosis related granuloma seen in the pathological examination
Electroanatomical mapping has been used in arrhythmogenic right ventricular cardiomyopathy with excellent correlation to cardiac MRI [16–19]. In addition, when endomyocardial regions of scar are identified, a bioptome can be directed to these areas for an improved diagnostic yield [22–24].
In order to perform voltage map guided biopsy, the region of scar is first identified with a roving catheter. The preferred site for biopsy is the right ventricular septum, which also represents the most common region affected by cardiac sarcoidosis. Biopsy of the epicardium and left ventricle have also been described. Again, a blind biopsy of the interventricular septum, as is common for the evaluation of other diffuse infiltrative cardiomyopathies, may not be appropriate for diagnosis of cardiac sarcoidosis given the heterogenous infiltration pattern described. Therefore, guidance with electroanatomical mapping may be more appropriate. In a study evaluating patients with right ventricular cardiomyopathy, EAM guided biopsy demonstrated a high rate of myocarditis mimicking ARVC [20]. Its use to specifically identify isolated cardiac sarcoidosis has also been demonstrated [21].
Conclusions
While the routine use of invasive testing cannot be supported based on current understanding, there are situations in which invasive testing provides clinically relevant information that can alter patient care that cannot be obtained with non-invasive testing. When there is a strong suspicion for cardiac sarcoidosis, especially isolated cardiac sarcoidosis, endomyocardial biopsy is appropriate and its yield may be increased by image- or electroanatomic- guided sampling.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


