Chapter 32 Invasive Mechanical Ventilation
Techniques
Modes of Invasive Mechanical Ventilation
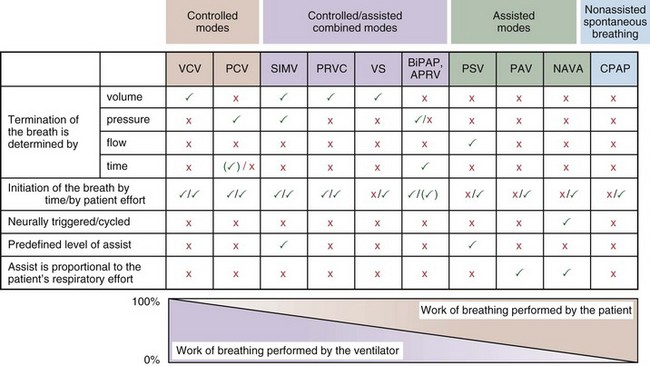
Most mechanical ventilators are capable of delivering ventilation in a fully controlled manner, to assist spontaneous breathing, and to facilitate nonassisted spontaneous breathing (Figure 32-1). Normally, the means by which inhalation is terminated (cycled off) is used to classify the ventilatory modes. Common mechanisms to cycle off the inhalation include volume, pressure, airflow, and time. Initiation of an inhalation (i.e., trigger-on) can be based either on predefined time intervals or on systems in which the patient’s inspiratory effort has to exceed a specific threshold. Most frequently, pneumatic systems are used that require a predefined, adjustable change in pressure or airflow within the ventilator circuit to trigger the initiation of an inhalation by the ventilator. Alternative trigger-on and cycling-off methods, such as changes in pleural pressure (PPL) (as can be assessed by esophageal pressure [PES] measurements) or changes in the amplitude of the diaphragm electrical activity (Edi), are currently entering clinical practice.
Modes That Target Volume or Pressure
Volume-Targeted (or -Controlled) Ventilation
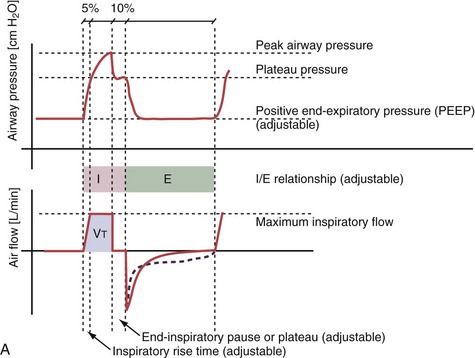
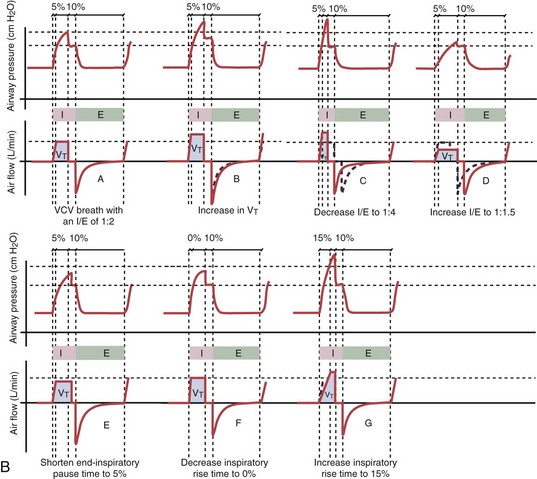
With time-cycled, volume-targeted (i.e., volume-controlled) ventilation (VCV), VT is preset, and the minute ventilation is guaranteed by the ventilator, depending on the ventilatory rate. Triggering delivery of additional breaths with the preset VT occurs when the patient’s breathing efforts exceed a caregiver-defined trigger-on threshold. Volume assist-control (A/C) refers to a variation of VCV in which the patient initiates delivery of a fixed VT by the ventilator, whereas a VCV backup rate ensures that the patient receives a minimum minute ventilation. Parameters that can be modified with most of the ventilators are the rate of inspiratory flow or ratio of the inspiratory to expiratory times (I/E ratio), the inspiratory rise time, the duration of the end-inspiratory pause time, and with some ventilators, the flow pattern during inhalation (Figure 32-2). The effects of changes in these parameters on the pressure and flow waveforms are demonstrated in Figure 32-2, B and C.
Pressure-Targeted (or -Controlled) Ventilation
With pressure-controlled ventilation (PCV), a maximum inspiratory pressure is targeted by the caregiver. The ventilator increases the pressure to this level during each breath. The flow pattern is decelerating; flow is high initially but decreases as the PVENT limit is approached. Inhalation is terminated when a predefined time limit or flow level is reached. The applied VT depends on the relative stiffness (elastance) of the respiratory system (e.g., VT is lower if pulmonary edema develops or if the patient contracts the abdominal or chest wall musculature) and on the resistance to airflow (e.g., VT will be lower if secretions markedly increase airway resistance). The adjustable parameters are the targeted PVENT, the inspiratory rise time, and, in some ventilators, the inspiratory time (Figure 32-3).
Modes That Deliver Assistance to Spontaneous Breathing
Patients often are ventilated with volume- or pressure-targeted modes (i.e., controlled ventilation) in the early phase of the disease process, whereas modes delivering assistance to spontaneous breathing usually are applied later. Assistance to spontaneous breathing is used to prevent respiratory muscle atrophy as well as to maintain physiologic feedback and intrinsic defense mechanisms, such as the Hering-Breuer reflex, based on the hypothesis that integration rather than abolition may help to minimize VILI. This approach should be better suited than “caregiver-controlled” mechanical ventilation to accommodate the typically rapid changes in lung mechanics and metabolic demands in critically ill patients. Ventilatory modes in which patients breathe spontaneously early in the course of the acute lung injury (ALI) process may have certain advantages, such as improved pulmonary ventilation-perfusion (V/Q) matching (Figure 32-4), increased oxygenation, preserved cardiac function, reduced need for excessive sedation, prevention of ventilation-associated respiratory muscle dysfunction, and ventilation at lower mean airway pressure, compared with controlled modes of ventilation. Induction of respiratory muscle fatigue or failure secondary to increased work of breathing is a potential disadvantage, but this frequently can be minimized with use of the appropriate level of positive end-expiratory pressure (PEEP) and adjustment of inspiratory flow rates. Of interest, recent data suggest that early neuromuscular blockade for 48 hours in patients with ALI may decrease mortality (see further on).
Pressure Support Ventilation
Pressure support ventilation is patient-triggered and, normally, flow-cycled, allowing the patient to actively control the start of each breath. Once the patient’s inspiratory effort exceeds the trigger-on threshold, a caregiver-defined level of PVENT is delivered to the airways (Figure 32-5, A). After a high initial airflow that is required to rapidly approach the targeted pressure, the airflow progressively decreases. Once a predefined percentage of the maximum inspiratory flow is reached, the ventilator terminates inhalation and opens the expiratory valve. Parameters that can be adjusted with pressure support ventilation are the trigger-on threshold, the inspiratory rise time, and the pressure level (see Figure 32-5, B). To better match termination of the inhalation with the patient’s individual demand, the cycling-off airflow threshold can be varied in some ventilators.
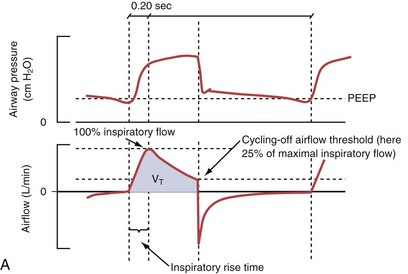
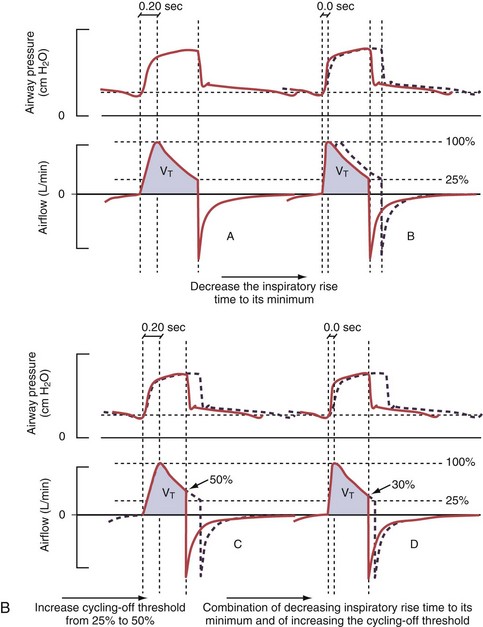
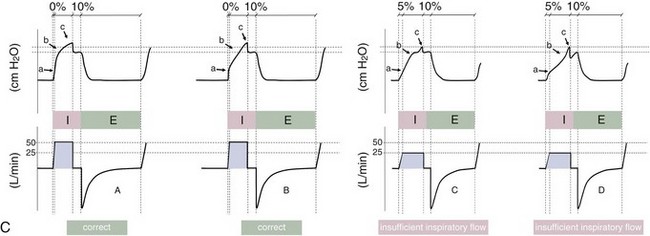
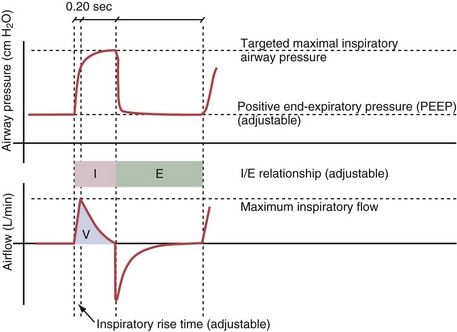
Figure 32-5 A, Pressure support ventilation (PSV). The delivered tidal volume (VT) is calculated by integrating the area under the flow curve, as indicated by the blue-shaded area. The inspiratory rise time defines how fast the maximal airflow (100%) is achieved. Thereafter, the inspiratory airflow continuously decreases because, once the pressure target is reached, maintaining this level requires progressively less air to flow into the lungs. As soon as the cycling-off airflow threshold (i.e., a preset percentage of maximal air flow) is reached, the ventilator ceases to deliver inspiratory flow, and the expiratory valve is opened to allow passive exhalation. B, Changes in the parameters of pressure support ventilation result in characteristic changes of the pressure and volume curves. Panels A to D demonstrate isolated changes of parameters assuming that all other ventilatory parameters and the mechanical characteristics of the respiratory system remain unchanged. A, PSV breath with an inspiratory rise time of 0.20 second. B, After reducing the inspiratory rise time to its minimum, the peak inspiratory flow and consequently also the cycling-off airflow threshold are both reached earlier. Decreasing the inspiratory rise time results in a shorter inspiratory time, while the VT remains unchanged. Note that although the inspiratory rise time is decreased to 0 seconds, the peak inspiratory flow is reached with a small delay. C, Increasing the cycling-off airflow threshold from 30% to 50% similarly shortens the inspiratory time; however, VT decreases in this case. D, A combination of a maximal decrease in the inspiratory time and a moderate increase in the cycling-off airflow threshold shortens the inspiratory time, whereas the loss in VT is only minimal. Such an approach can be used to achieve a prolongation of the expiratory time in patients at risk for dynamic hyperinflation because of expiratory flow limitation (e.g., patients with COPD) (see also Figure 32-19). COPD, chronic obstructive pulmonary disease.
Difficulties with Conventional Modes of Assistance to Spontaneous Breathing
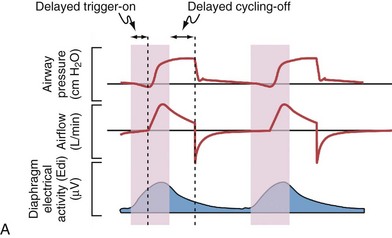
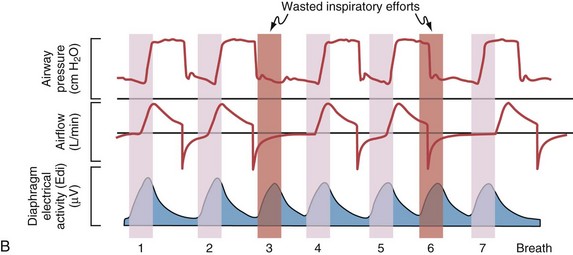
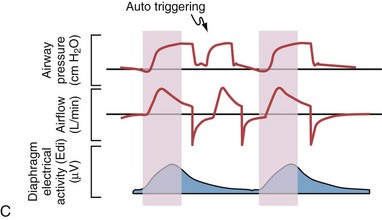
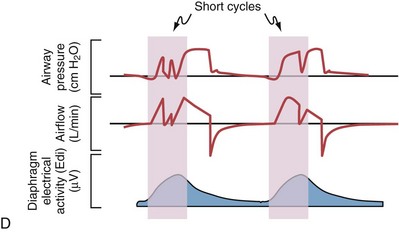
Although important improvements have been made in the trigger-on characteristics and the cycling-off characteristics of ventilators, ideal synchrony between the ventilator and the patient has not been achieved with most modes of ventilation, and patient-ventilator asynchrony is common (Figure 32-6). Patient-ventilator asynchrony may result in increased inspiratory and expiratory muscle activity and may introduce an unnecessary burden, in terms of work of breathing, in patients whose respiratory muscles are already under stress. Technologies that allow delivery of assistance in proportion to the patient’s demand on a breath-by-breath basis have only recently been developed (e.g., proportional assist ventilation [PAV] and neurally adjusted ventilatory assist [NAVA], as described later on).
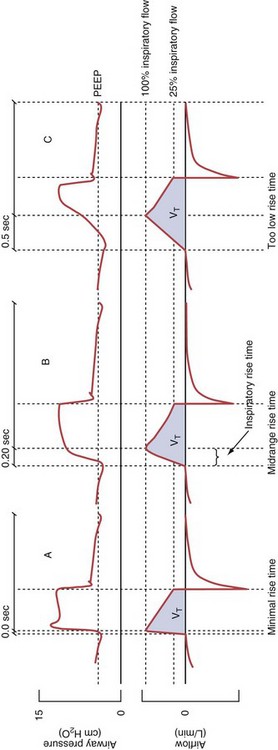
Variables that can be adjusted to improve synchrony between the patient and the ventilator include the trigger-on threshold, the inspiratory rise time or flow rate, and the cycling-off airflow threshold. The mechanisms used to initiate a breath (trigger-on) detect changes in airflow or pressure in the ventilatory circuit. Hence, negative deflections of short duration (pneumatic trigger mechanisms) may be detectable in the airway pressure and/or flow tracings when a breath is triggered by the patient’s effort. Delivery of the assistance is terminated either after a predefined time has elapsed (time-cycled) or after a prespecified cycling-off airflow threshold (flow-cycled) has been reached (see Figure 32-5). Rise time refers to the time required by the ventilator to increase the inspiratory airflow from zero to peak. As demonstrated in Figure 32-7, the rise time changes the slope of the increase in pressure during early inspiration. Generally, rise time (or the inspiratory flow pattern) should be set to ensure that air is delivered rapidly (fast increase in airway pressure) after initiation of a breath. By establishing an optimal inspiratory rise time, synchrony to the patient’s respiratory demand can be optimized and work of breathing can be reduced.
Proportional Assist Ventilation
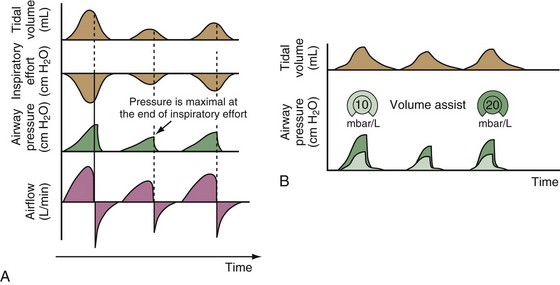
PAV, the first patient-triggered mode that adapted the level of assist to the patient’s inspiratory effort, was introduced in 1987. With PAV, the ventilator delivers positive pressure throughout inspiration in proportion to the inspiratory airflow and volume generated by the patient (Figure 32-8). The magnitude of unloading is based on measuring elastance and resistance of the respiratory system. Whereas with conventional modes of ventilation the VT or the delivered PVENT is relatively constant from breath to breath, with PAV only the relationship between delivered PVENT and the inspiratory effort of the patient is constant, whereas VT and the delivered PVENT become dependent variables. Although PAV requires that the patient always assume a portion of the respiratory work, this mode has been demonstrated to effectively unload the respiratory muscles.
Neurally Adjusted Ventilatory Assist
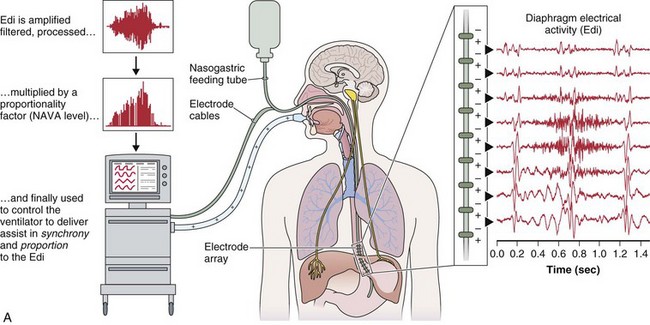
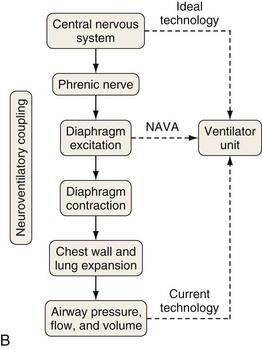
A relatively new strategy of mechanical ventilation, NAVA, uses the Edi to control the ventilator (Figure 32-9). Because breathing signals originate from the brain and reach the diaphragm by way of the phrenic nerves, Edi represents the neural respiratory effort with respect to both timing and amplitude. During NAVA, positive pressure is applied to the airway opening in direct proportion to the Edi amplitude, so defining a target pressure or volume is not required. The patient’s respiratory control mechanisms, including feedback from mechanoreceptors and chemoreceptors, adjust the Edi and thereby regulate the pressure and delivered volume. Animal data and a number of clinical studies suggest that NAVA is applicable in the ICU environment, efficiently delivers assistance synchronous to the subject’s demand, unloads the respiratory muscles, maintains gas exchange, and preserves cardiac performance during invasive ventilation and also during noninvasive ventilation even with use of an excessively leaky interface. Simultaneous measurement of the Edi (which reflects the patient’s neurally generated effort) and the delivered assist allows monitoring the patient’s ability to translate a neural effort into ventilation, referred to as neuroventilatory efficiency. NAVA does not require measurement of respiratory system mechanics, and runaway phenomena are unlikely to occur.
Combined Modes
Airway Pressure Release Ventilation
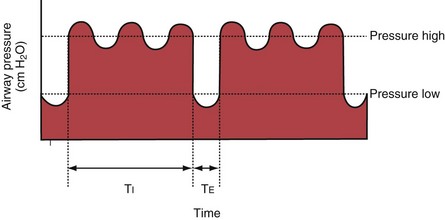
With airway pressure release ventilation (APRV) (Figure 32-10), the pressure in the ventilator circuit alternates between a high and a lower level (normally the higher pressure level is of longer duration than the lower pressure level) and spontaneous breathing is allowed in any phase of the cycle. The high- and low-pressure levels, the rate of change between the two levels, the respiratory system compliance, and the airway resistance to flow are the main determinants of the “mechanical ventilation” portion with APRV, whereas the complementary “spontaneous breathing” portion mainly depends on the patient’s respiratory drive. In contrast with continuous positive airway pressure (CPAP), APRV interrupts PVENT briefly to augment spontaneous minute ventilation and thereby increases alveolar ventilation and CO2 removal without increasing the work of breathing. Spontaneous efforts during APRV are not actively assisted except for those breaths that happen to occur during the change from the lower to the upper pressure level. Total minute ventilation with APRV is the sum of the mechanical, pressure-controlled ventilation and the complementary spontaneous breathing. APRV without spontaneous breathing is essentially the same as PCV.

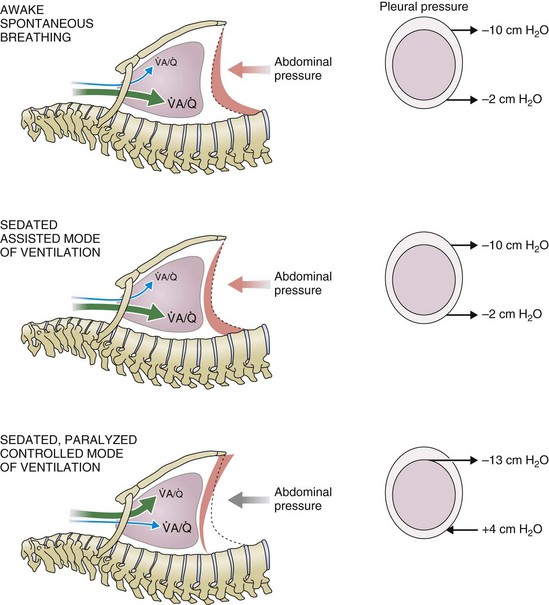
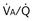 ) distribution during spontaneous breathing and during mechanical ventilation. In a classic article, Froese and Bryan demonstrated that with spontaneous breathing (in either an awake or sedated patient), dorsal excursions of the diaphragm were more pronounced than ventral excursion. The active diaphragm generates a greater negative pressure in the dorsal pleural space, thereby increasing the pressure gradient between the central airways and the pleural space (transpulmonary pressure); this promotes alveolar recruitment and ventilation of the dependent lung regions. Ventilation of dependent, usually well-perfused parts of the lungs, along with an increase in blood flow to previously minimally perfused or nonperfused areas, helps to convert shunt units to units with normal
) distribution during spontaneous breathing and during mechanical ventilation. In a classic article, Froese and Bryan demonstrated that with spontaneous breathing (in either an awake or sedated patient), dorsal excursions of the diaphragm were more pronounced than ventral excursion. The active diaphragm generates a greater negative pressure in the dorsal pleural space, thereby increasing the pressure gradient between the central airways and the pleural space (transpulmonary pressure); this promotes alveolar recruitment and ventilation of the dependent lung regions. Ventilation of dependent, usually well-perfused parts of the lungs, along with an increase in blood flow to previously minimally perfused or nonperfused areas, helps to convert shunt units to units with normal 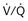 distribution, thereby increasing oxygen content of arterial blood, and to lower pulmonary vascular resistance. During application of positive-pressure ventilation with inactive or only minimally active inspiratory muscles (e.g., with neuromuscular blockade or with hyperventilation), gas is preferentially distributed toward the ventral regions of the lungs, where the impedance to airflow is lower than in the dependent, partially atelectatic regions. Generation of positive pressure in the dorsal pleura promotes collapse of alveoli in the dorsal lung regions and the
distribution, thereby increasing oxygen content of arterial blood, and to lower pulmonary vascular resistance. During application of positive-pressure ventilation with inactive or only minimally active inspiratory muscles (e.g., with neuromuscular blockade or with hyperventilation), gas is preferentially distributed toward the ventral regions of the lungs, where the impedance to airflow is lower than in the dependent, partially atelectatic regions. Generation of positive pressure in the dorsal pleura promotes collapse of alveoli in the dorsal lung regions and the 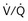 mismatch becomes worse.
mismatch becomes worse.