Fig. 1.1
Transferring knowledge from basic science to the clinical area (Reproduced from Antoniades [30] with permission)
Again it must be pointed out that these are definitions given by experienced investigators which are similar among them, but differ somewhat according to their creators’ backgrounds and research interests.
Hecker and Birla [6] stress that strategy in research consists of two differing directions: The more frequent, especially, I would think, in well organized departments in the academic setting, is the Top Down Strategy, in which a vision is set. To ensure successful implementation of this vision, one must set objectives to be attained, and research activities to be undertaken.
The same authors describe that in the bottom-up approach, the outcome of experimentation dictates the overall strategic research direction.
The finding by Murry et al. [31] of ischemic preconditioning is such an example. This latter approach allows for a dynamic environment and promotes creativity. However, the authors very thoughtfully point out [6] that integration of the two models is quite frequent and can lead to the greatest success.
Of course just establishing definitions for these research patterns does not automatically ensure investigational bliss and clinical success.
1.5 Blocks to Translational Research
Lauer and Scarlatos [23], reiterate the three translational blocks or obstacles identified by the Institute of Medicine Clinical Research Roundtable [22], pertaining to T1, T2 and T3.
Chautard et al. [32] have further defined the first block as this limiting translation of new knowledge into clinical practice and health decision making towards improved health. As components of the former they cite lack of willing participants, regulatory burdens, fragmented infrastructure, incompatible databases, and lack of qualified investigators. As components of the latter, they mention career disincentives, practice limitations, high research costs and lack of funding. A T3 block can be regarded as limitation of dissemination into practice, communities, and large populations.
Here it must be stressed that the NIH itself points out that funding between T1 and T2 is not evenly balanced, to some extent explaining the block between these stage.
Thus, according to some 2007 data the NIH allots 13 billion dollars to Basic Research but only 787 millions to Health Services Research. Researchers from the National Cancer Institute stresses another important point, that research dissemination and diffusion are costly by themselves [33].
Hecker and Birla [6] give two interesting additional definition pertaining to research.
Thus they propose two more entities:
(a)
Development is the systematic use of the knowledge or understanding gained from research of practical experience directed toward the production or significant improvement of useful products.
(b)
Applied Research is the acquisition of knowledge or understanding to meet a specific recognized need”.
According to Vanovar Bush [5] the function of applied research is to give answers which Basic Research cannot provide.
Another goal of TR which the European Society of Cardiology specifically endorses is innovation, which should be the strong point of discovery.
Deborah Zucker [34] describes that innovation connotes something novel, original, visionary, and hopefully improved. It necessitates the generation of new ideas and hypotheses.
These newer notions signify the quest for the attainment of results and not just the acquisition of new data, which is becoming more pressing with time.
The question then arises, by what measures can TR be carried out and produce favorable results for the improvement of the care of the individual patient and the human populations?
1.6 Organizational Aspects
The evolution towards organizing TR in the United States is interesting and didactic, as presented by Robertson and Williams [19]. They describe how the General Clinical Research Centers (GCRCs) evolved to the Clinical and Translational Science Award (CTSA) program in 2005.
They give a draft of the conceptual framework of the National CTSA Consortium.
The CTSA consortium comprises numerous Academic Health Centers (AHCs) in various states.
From their Fig. 1.1 one can readily appreciate the complexity but also the scope of these clinical and Translational Research Institutes.
Recently, Gordon R Bernard [35] described the establishment of a new NIH center, the National Center for Advancing Translational Services (NCATS) with a budget of $576.5 millions, comprising the Clinical and Translational Science Award (CTSA) program, a consortium of 60 sites.
1.6.1 The Value of Collaborations-Paradigms
First, the necessity of collaborations must be realized and appreciated. Thus, Lauer and Scarlatos [23] point out in the Progenitor Cell Biology Consortium that collaborations among institutions are essential. They describe the path from discovery (presumably produced through Basic Research), as producing early (pilot) T1 studies and ancillary studies towards yielding clinical applications.
In the Basic Research component, this Consortium is the main vehicle. A large number of Research Units is coordinated by the Administrative Coordinating Center, which is the University of Maryland, Baltimore.
As regards clinical applications, the Cardiovascular Cell Therapy Research Network consists of important clinical departments: i.e. the Vanderbilt University, the University of Florida, the Cleveland Clinic and the Texas Heart Institute.
A similar paradigm is offered by the Pediatric Cardiovascular Translational Bench to Bassinet Program.
Again one can mark three stages:
Discovery is managed by the Cardiovascular Development Consortium, managed by four research Units (Gladstone, Harvard, Pittsburgh, Utah).
Early T1 is effected by the Pediatric Cardiac Genomics Consortium, comprising outstanding units (Boston, Columbia, Philadelphia, Mt. Sinai, Yale).
Finally the Clinical Application part is implemented by eight clinical sites which constitute the Pediatric Heart Network, a multicenter approach.
Bernard [35] also stresses the importance of collaboration to enable multi-site translational science.
This accent on collaboration brings into focus a consideration by Hecker and Birla [6]: They stress that many investigators or departments have a conception of territorial belonging or ownership of an idea. This narrow outlook is counter-productive and hampers collaboration.
1.6.2 Training Translational Research Scientists
This brings into focus the question: Can and should TR be taught? The answer to both is yes. Before considering how, let us outline the requirements of becoming a Translational Researcher.
In the aforementioned excellent and detailed book, K. E Hartman, E. Heitman, and N. Brown [36] devote a chapter on recommended knowledge bases for T1 and T2 Translational Research. Not surprisingly the requirements for the former discipline are simpler.
Thus, they describe that for T1, recommended knowledge base includes:
Human and molecular biology and pathophysiology, animal models, and laboratory techniques.
The TR translational science components include genomics, proteomics, imaging technology, biomarker development, biomedical informatics, dataset acquisition, and management.
Finally, core competences additionally include biostatistics, epidemiology, study design, research ethics, writing and communication.
For a T2 career, health care epidemiology and other care epidemiology methods are additionally required.
The authors point out that guidelines exist for the education in Clinical and Translational Research of students since 2008, and residents since 2007. They point out that although the mentored/apprentice research prototype still applies, participation into a core didactic curriculum is essential, as well as participation in formal career and leadership development activities. They also describe that some academic medical centers have established training programs in Clinical and early Translational Research, are their majority directed at MDs in post-doctoral training. These curricula vary widely. Moreover, for more advanced training a few programs offer PhDs in Clinical and TR per se.
Barry Coller according to David Schteintgart [8] proposes that the Basic and Clinical Researchers represent two separate cultures.
Similar specifications are proposed by many authors.
Thus, according to Williams and Robertson [37], a TR team should include:
Laboratory-based investigators
Clinical investigators
Statisticians
Data managers
Research nurses and coordinators
Research Pharmacists
The same authors, propose that the twenty first century human research laboratory should have the following picture (Fig. 1.2).
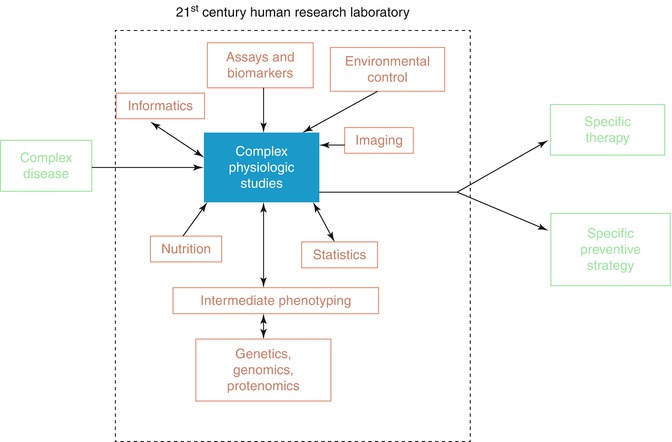

Fig. 1.2
The twenty-first century human translational investigator’s laboratory (Reprinted from: Williams and Robertson [37], with permission of Elsevier)
According to Schteingart [8] an appropriate collaborative model should encourage partnerships between researchers, practitioners and people skilled in translation.
A proposed concept of who is and who is not a translational investigator is given by Williams and Robertson [37] as adapted from Lee M Nadler, who gave some further thoughts on this subject in 2007 [38].
To produce such results many medical schools are proposing various teaching courses for producing this type of researchers.
However, it must be realized that there is no formal training to produce a translational researcher. David Schteingart [8] proposes how to train such scientists:
For those coming from a clinical background he proposes courses and seminars in genetics, molecular biology, computational biology, molecular imaging, epidemiology, and therapeutics.
From those coming from a basic laboratory, he proposes clinical immersion and to learn principles of clinical research. Furthermore, he recommends:
Translational Research training principles
Multidisciplinary training
Training customized to an individual’s background and skills
Mentoring by mentoring committees with diverse areas of expertise
Adaptation to working in multidisciplinary teams
The same author thoroughly accepts that many challenges remain:
Translational Research training challenges
Career paths uncertain
Limited number of well trained mentors
Competences still being developed
Unclear role in the academic research environment
No specific evaluation criteria
McGartland Rubio et al. [27] discuss the implications for the design of training programs. They stress that since there exists a great diversity of educational background it may be necessary to design a separate curriculum for every individual trainee. They add that those with basic research background will need to acquire practice in clinical sciences and practice, while clinical background trainees will need additional exposure to basic science. They also stress the importance of mentoring, which will be further discussed. This aspect they consider demanding but highly rewarding.
Bernard [35] stresses the importance of educating and training scientists in clinical and translational research.
At the Biomedical Research Foundation of the Academy of Athens (BRFAA), we are conducting a yearly course, which actually gave the momentus for this book.
1.6.2.1 Leadership
It should be appreciated that, as in all fields of scientific endeavor, correct mentoring of the younger investigators is important and essential. The word “mentor” emerges from Homer’s Odyssey. The Goddess Athena is disguised as Mentis or Mentor, a friend of Ulysses, who offers to guide his son Telemachus in a journey to gather news about his father.
A strategy in any department cannot be fruitful unless a sense of leadership is cultivated. Leadership develops leaders at all levels, with as a result exponential growth according to Hecker and Birla [6].
The same authors point out that success in scientific research can be determined by both tangible and, equally important, intangible factors, As tangible factors one can enumerate publications, impact factor, citations, Hirsch index, invited lectures and articles, positions in committees, grants, patents, and finally return on investment stock prices, etc.
Intangible rewards are to this and to many authors more important and long-lasting. Hecker and Birla [6] describe them as creating a positive work environment momentum and managing innovation. Additionally, these authors point out that leadership is different from management:
The latter term is impersonal and concerns resource allocation and accountability, while the former concerns the ability to work and connect with people and to convince them to implement change.
In this sense, in an inspiring article, Verkoeijen and Tabbers point out that good research requires productive theories and guidelines [39].
The PLoS Medicine Editors Tikki Pang and Robert F. Terry stress that in the twenty first century it seems astonishing that decisions on healthcare are still made without a solid grounding one research evidence [40].
1.6.3 The Blocks and Problems
Here, it should be stressed that the effort toward advancing T1 are well delineated and adequately stressed.
However, T2 is also gradually acquiring greater importance, because it is realized that many blocks in its implementation remain.
White et al. [41] and Green et al. [42] point out that the great majority of patients are treated in primary care centers.
Thus, we do not often get a real picture of everyday medicine if we only take into account the academic clinical centers, in which an “artificial” atmosphere may be said to exist.
To counteract this problem, has been created, which according to Westfall et al. [24], the NIH Roadmap tries to connect major academic laboratories to physicians and primary physician offices.
The authors further point out that since recommendations and guidelines are created from relatively small groups in selected tertiary centers their applicability may be limited and not reflect real world day –to-day practice.
Martin et al. [43] very recently point out that apart from the above mentioned factors, additional patient and trial barriers exist. Patient-specific factors were older age, out-of-state residence and female gender Trial specific barriers exist were intensive trial-related testing and long anticipation (>6 months).
The first item mentioned, which is essential to the success of clinical trials is simplicity, the principle of KISS (keep it simple and stupid), since intensive testing militates against adherence.
These considerations become more important currently because of the increasing cost and difficulty of conducting research; a main consideration is how to produce worthwhile results.
However, many researchers Dirk Brutsaert [44] being a distinguished example, question the future of clinical trials, in this instance in chronic heart failure. According to him, most single target-oriented clinical trials are doomed to fail. He advocates that we should incorporate Genome Wide Association Studies (GWAs), and research should be multiscaled- and multitarget-based and network medicine oriented. This is in contrast to the vast majority of multicenter clinical trials. Robert Calif [45] underlines the difficulties facing the planners of clinical trials. He points out that unintended biological targets are common (for example intracranial haemorrhage in thrombolysis for acute myocardial infarction), and that interactions among therapies and long-term effects are unpredictable. He points out to the necessity of embedding clinical trials within disease registries [46].
The Multicenter Research Group [47] realizing the shortcomings and mounting expenses of collaborative clinical trials have advanced many proposals. In accordance with the previous concerns about too much simplicity, they propose the evaluation of the effect of combing different therapies that target diverse pathways or mechanisms in complex medical disorders.
Very recently Sipido et al. [48] propose a clinical trial checklist:
1.
Robust number of observations to ensure data care reliable.
2.
Randomization and blinded observations.
3.




Correct data processing.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


