The aim of this study was to investigate the relation between intravascular ultrasound (IVUS) findings and the no-reflow phenomenon and long-term outcome after percutaneous coronary intervention (PCI) of saphenous vein graft (SVG) lesions. No reflow was defined as Thrombolysis In Myocardial Infarction grade 0, 1, or 2 flow after PCI. Of 311 patients who underwent IVUS before and after stenting, no reflow was observed in 39 patients (13%). Degenerated SVG (62% vs 36%, p = 0.002), IVUS-detected intraluminal mass (82% vs 43%, p <0.001), culprit lesion multiple plaque ruptures (23% vs 6%, p <0.001), and tissue prolapse (51% vs 35%, p = 0.043) were observed more frequently in patients with no reflow. In multivariate logistic regression analysis, an intraluminal mass (odds ratio [OR] 4.84, 95% confidence interval [CI] 1.98 to 10.49, p = 0.001), culprit lesion multiple plaque ruptures (OR 3.46, 95% CI 1.46 to 8.41, p = 0.014), and degenerated SVGs (OR 3.17, 95% CI 1.17 to 6.56, p = 0.024) were the independent predictors of no reflow after PCI. At 5-year clinical follow-up, rates of death (14, 36%, vs 55, 20%, p = 0.036) and myocardial infarction (13, 33%, vs 52, 19%, p = 0.039) were significantly higher in the no-reflow group. However, rate of target vessel revascularization was not significantly different between the 2 groups (15, 38%, vs 90, 33%, p = 0.3). IVUS-detected intraluminal mass, multiple plaque ruptures, and degenerated SVGs were associated with no reflow in SVG lesions after PCI. In conclusion, no reflow was associated with poor long-term clinical outcomes after PCI for SVG lesions.
Percutaneous coronary intervention (PCI) for saphenous vein graft (SVG) disease is limited by distal embolization that can cause creatine kinase-MB increases or no reflow, the occurrence of which is associated with a 15% rate of mortality and a 31% rate of acute myocardial infarction. It would be valuable to be able to predict high-risk SVG lesions. Although several studies have reported the intravascular ultrasound (IVUS) predictors of no reflow after PCI for native coronary arteries, to the best of our knowledge no study has examined the relation between IVUS findings and no reflow after PCI for SVG lesions. Therefore, the purpose of the present study was to attempt to identify IVUS findings before and after PCI that are predictive of no reflow after PCI for SVG disease.
Methods
We reviewed 311 patients who underwent IVUS-guided PCI for 311 SVG lesions in the Washington Hospital Center from June 2000 through December 2005. We excluded patients with restenotic SVG lesions, patients treated for stent thrombosis, patients with cardiogenic shock, and patients in whom adequate IVUS images could not be obtained. All patients were treated with stent implantation: 153 patients with sirolimus-eluting stents, 56 patients with paclitaxel-eluting stents, and 102 patients with bare-metal stents. The protocol was approved by the institutional review board. Hospital records of all patients were reviewed to obtain clinical demographics and medical history.
No reflow was defined as Thrombolysis In Myocardial Infarction (TIMI) grade 0, 1, or 2 flow after PCI in the absence of mechanical obstruction. Normal reflow was defined as TIMI grade 3 flow. Patients were divided into a no-reflow group (n = 39) and a normal-reflow group (n = 272). If TIMI flow after PCI was grade 0, 1, or 2 in the absence of angiographic stenosis, repeat IVUS was performed to exclude the possibility of mechanical vessel obstruction. Degenerated SVG was defined as luminal irregularities or ectasia involving >50% of its total length. Angiographic thrombus was defined as a discrete intraluminal filling defect with defined borders largely separated from the adjacent wall.
Quantitative analysis (CAAS II, Pie Medical, the Netherlands) was performed using standard protocols. With the outer diameter of the contrast-filled catheter as the calibration standard, the reference diameter and minimal lumen diameter were measured in diastolic frames from orthogonal projections. Perfusion was evaluated according to TIMI criteria.
All IVUS examinations were performed after intra-SVG administration of nitroglycerin 200 μg using a commercially available IVUS system (Boston Scientific Corporation/SCIMed, Minneapolis, Minnesota). The IVUS catheter was advanced distal to the target lesion, and imaging was performed retrograde to the aorto-ostial junction at an automatic pullback speed of 0.5 mm/s.
Quantitative and qualitative analyses were performed according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement, and Reporting of Intravascular ultrasound Studies. Before PCI, we measured SVG and lumen cross-sectional area (CSA) using planimetry software (TapeMeasure, Indec Systems, Inc., Mountain View, California). The SVG CSA was measured by tracing the outer border of the entire vein graft. Plaque CSA was calculated as SVG CSA minus lumen CSA, and plaque burden was calculated as plaque CSA divided by SVG CSA. The lesion site was the image slice with the smallest lumen; if there were multiple image slices with the same minimum lumen CSA, then the image slice with the largest SVG and plaque CSAs was measured. Remodeling index was the ratio of lesion site SVG CSA divided by the average of the proximal and distal reference SVG CSAs. Positive remodeling was defined as a remodeling index >1.05, intermediate remodeling as a remodeling index 0.95 to 1.05, and negative remodeling as a remodeling index <0.95.
An intraluminal mass had a layered lobulated appearance, evidence of blood flow (microchannels) within the mass, and speckling or scintillation (similar to thrombus in native coronary arteries; Figure 1 ) . A ruptured plaque contained a cavity that communicated with the lumen with an overlying residual fibrous cap fragment ( Figure 1 ). Rupture sites separated by a length of SVG containing smooth lumen contours without cavities were considered to represent different plaque ruptures. A lipid pool-like image was defined as hypoechoic or echolucent material covered with a hyperechoic layer. Soft plaque was less bright than the adventitia, fibrotic plaque was as bright as or brighter than the adventitia without acoustic shadowing, and calcific plaque was brighter than the adventitia with acoustic shadowing. When there was no dominant plaque composition, the plaque was classified as mixed.
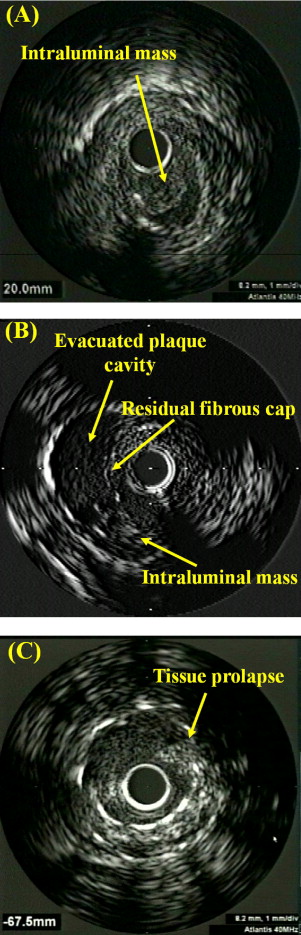
After PCI we measured the minimum stent CSA. Stent expansion was calculated as minimum stent CSA divided by mean reference lumen CSA. Tissue prolapse was defined as tissue protrusion through the stent strut after the procedure ( Figure 1 ), and the volume of tissue prolapse was calculated by subtracting lumen volume from stent volume.
We examined major adverse cardiac events at 5 years according to the presence or absence of no reflow after PCI for SVG lesions. Death included mortality from all causes. Myocardial infarction was defined as (1) evolutionary ST-segment elevation, development of new Q waves in ≥2 contiguous electrocardiographic leads, or new left bundle branch block patterns on electrocardiogram or (2) biochemical evidence of myocardial necrosis, manifested as (a) creatine kinase-MB ≥3 times the upper limit of normal or, if creatine kinase-MB was not available, (b) total creatine kinase ≥3 times the upper limit of normal, or (c) troponin value above the upper limit of normal. Target vessel revascularization was defined as repeat revascularization involving the initially treated SVG. Native vessel target vessel revascularization was not included in the target vessel revascularization end point.
Statistical analysis was performed using SAS 9.1 (SAS Institute, Cary, North Carolina). Continuous variables were presented as mean ± 1 SD; comparisons were conducted by Student’s t test or nonparametric Wilcoxon test if normality assumption was violated. Discrete variables were presented as percentages and relative frequencies; comparisons were conducted by chi-square test or Fisher’s exact test as appropriate. Logistic regression analysis was used to identify independent predictors of no-reflow phenomenon. A p value <0.05 was considered statistically significant.
Results
Of 311 patients studied with IVUS before PCI, no reflow was observed in 39 patients (13%) after PCI. Baseline characteristics are listed in Table 1 . PCI for myocardial infarction compared to that for angina was more frequently associated with no reflow. The PercuSurge Guardwire Plus System (Medtronic AVE, Sunnyvale, California) was used in 80 patients, the Angioguard Filter System (Cordis, Miami, Florida) was used in 25 patients, and the Filterwire EX (Boston Scientific EPI, Santa Clara, California) was used in 20 patients.
| Variable | No Reflow (n = 39) | Normal Reflow (n = 272) | p Value |
|---|---|---|---|
| Age (years) | 71.4 ± 10.9 | 69.1 ± 10.3 | 0.19 |
| Men | 33 (85%) | 201 (74%) | 0.15 |
| Clinical presentation | 0.034 | ||
| Stable angina pectoris | 10 (26%) | 80 (29%) | |
| Unstable angina pectoris | 20 (51%) | 156 (57%) | |
| Non–ST-segment elevation myocardial infarction | 8 (21%) | 36 (13%) | |
| ST-segment elevation myocardial infarction | 1 (3%) | 0 (0%) | |
| Diabetes mellitus | 15 (39%) | 102 (38%) | 0.9 |
| Hypertension | 27 (69%) | 200 (74%) | 0.6 |
| Smoker | 17 (44%) | 109 (40%) | 0.7 |
| Hypercholesterolemia (total cholesterol >220 mg/dl) | 33 (85%) | 202 (74%) | 0.16 |
| Years after saphenous vein graft surgery | 11.6 ± 7.3 | 11.1 ± 6.5 | 0.6 |
| Ejection fraction (%) | 42 ± 10 | 51 ± 9 | 0.16 |
| Distal protection device use | 19 (49%) | 106 (39%) | 0.2 |
| Bivalirudin use | 33 (85%) | 236 (87%) | 0.7 |
| Glycoprotein IIb/IIIa inhibitor use | 11 (28%) | 59 (22%) | 0.4 |
| Preintervention clopidogrel loading | 17 (44%) | 132 (49%) | 0.6 |
| Postintervention creatine kinase-MB (ng/ml) | 32.1 ± 104.9 | 4.1 ± 14.4 | <0.001 |
Angiographic findings and procedural results are presented in Table 2 . Patients with no reflow presented with a lower TIMI flow grade before PCI. There were no significant differences in TIMI flow grade before PCI according to use of distal protection devices (TIMI 0 flow, 4% vs 6%; TIMI 1 flow, 1% vs 0%; TIMI 2 flow, 12% vs 14%; TIMI 3 flow, 83% vs 80% in no use vs use of distal protection devices, p = 0.5). There were no significant differences in TIMI flow grade after PCI according to use of distal protection devices (TIMI 0 flow, 1% vs 0%; TIMI 1 flow, 0% vs 2%; TIMI 2 flow, 10% vs 14%; TIMI 3 flow, 89% vs 85% in no use vs use of distal protection devices, p = 0.2). Degenerated SVGs and angiographic thrombus were observed more significantly in the no-reflow group.
| Variable | No Reflow (n = 39) | Normal Reflow (n = 272) | p Value |
|---|---|---|---|
| Narrowed saphenous vein graft to | 0.4 | ||
| Left anterior descending coronary artery | 5 (13%) | 58 (21%) | |
| Left circumflex coronary artery | 18 (46%) | 99 (36%) | |
| Right coronary artery | 16 (41%) | 115 (42%) | |
| Lesion site | 0.2 | ||
| Ostium | 3 (8%) | 61 (22%) | |
| Proximal | 17 (44%) | 82 (30%) | |
| Middle | 9 (23%) | 60 (22%) | |
| Distal | 10 (26%) | 69 (25%) | |
| Preintervention Thrombolysis In Myocardial Infarction flow grade | <0.001 | ||
| 0 | 5 (13%) | 11 (4%) | |
| 1 | 2 (5%) | 0 (0%) | |
| 2 | 14 (36%) | 25 (9%) | |
| 3 | 18 (46%) | 236 (87%) | |
| Degenerated saphenous vein graft | 24 (62%) | 97 (36%) | 0.002 |
| Thrombus | 16 (41%) | 57 (21%) | 0.006 |
| Stent type | 0.3 | ||
| Sirolimus-eluting stent | 15 (39%) | 138 (51%) | |
| Paclitaxel-eluting stent | 10 (26%) | 46 (17%) | |
| Bare-metal stent | 14 (36%) | 88 (32%) | |
| Stent diameter (mm) | 3.47 ± 0.69 | 3.31 ± 0.49 | 0.077 |
| Stent length (mm) | 22.6 ± 7.5 | 20.7 ± 7.8 | 0.16 |
| Direct stenting | 24 (62%) | 189 (70%) | 0.3 |
| Inflation pressure (atm) | 13.6 ± 3.6 | 13.6 ± 3.0 | 1.0 |
| Adjunct balloon | 14 (36%) | 115 (42%) | 0.4 |
| Inflation pressure (atm) | 16.7 ± 3.6 | 16.1 ± 3.5 | 0.5 |
| Postintervention Thrombolysis In Myocardial Infarction flow grade | <0.001 | ||
| 0 | 1 (3%) | 0 (0%) | |
| 1 | 2 (5%) | 0 (0%) | |
| 2 | 36 (93%) | 0 (0%) | |
| 3 | 0 (0%) | 272 (100%) | |
| Reference diameter (mm) | 3.35 ± 0.69 | 3.33 ± 0.63 | 0.7 |
| Preintervention minimal lumen diameter (mm) | 0.78 ± 0.41 | 0.84 ± 0.46 | 0.089 |
| Postintervention minimal lumen diameter (mm) | 3.25 ± 0.55 | 3.12 ± 0.69 | 0.061 |
IVUS results are presented in Table 3 . Lesion site lumen CSA was significantly smaller, plaque burden and remodeling index were significantly greater, and lesion length was significantly longer in the no-reflow group. Presence of an intraluminal mass, culprit lesion plaque rupture and multiple plaque ruptures, lipid pool-like image, soft plaque, and positive remodeling were significantly more common in patients with no reflow. Plaque cavity CSA was significantly greater in the no-reflow group (3.14 ± 0.62 vs 2.47 ± 1.29 mm 2 , p = 0.008). Minimum stent CSA and stent expansion were significantly greater in the no-reflow group. Tissue prolapse after PCI was observed more frequently and maximum tissue prolapse area and tissue prolapse volume were significantly greater in patients with no reflow.
| Variable | No Reflow (n = 39) | Normal Reflow (n = 272) | p Value |
|---|---|---|---|
| Reference | |||
| Saphenous vein graft cross-sectional area (mm 2 ) | 13.9 ± 5.6 | 14.1 ± 5.1 | 0.9 |
| Lumen cross-sectional area (mm 2 ) | 9.3 ± 3.9 | 9.3 ± 3.1 | 1.0 |
| Plaque cross-sectional area (mm 2 ) | 4.5 ± 2.8 | 4.7 ± 3.3 | 0.6 |
| Plaque burden (%) | 31.0 ± 12.3 | 32.0 ± 12.2 | 0.6 |
| Lesion site | |||
| Saphenous vein graft cross-sectional area (mm 2 ) | 14.7 ± 5.6 | 14.2 ± 5.3 | 0.5 |
| Lumen cross-sectional area (mm 2 ) | 2.5 ± 1.1 | 3.2 ± 1.2 | 0.001 |
| Plaque cross-sectional area (mm 2 ) | 12.3 ± 5.6 | 11.0 ± 5.1 | 0.15 |
| Plaque burden (%) | 80.6 ± 11.4 | 75.4 ± 10.4 | 0.004 |
| Lesion length (mm) | 17.3 ± 9.1 | 13.8 ± 6.8 | 0.026 |
| Intraluminal mass | 32 (82%) | 117 (43%) | <0.001 |
| Plaque rupture | 17 (44%) | 44 (16%) | <0.001 |
| Multiple plaque ruptures | 9 (23%) | 15 (6%) | <0.001 |
| Lipid pool-like image | 12 (31%) | 46 (17%) | 0.038 |
| Plaque composition | 0.004 | ||
| Soft | 23 (59%) | 116 (43%) | |
| Fibrotic | 11 (28%) | 123 (45%) | |
| Calcific | 2 (5%) | 30 (11%) | |
| Mixed | 3 (8%) | 3 (1%) | |
| Remodeling index | 1.07 ± 0.16 | 1.01 ± 0.15 | 0.019 |
| Remodeling pattern | 0.042 | ||
| Positive | 21 (54%) | 92 (34%) | |
| Intermediate | 11 (28%) | 93 (34%) | |
| Negative | 7 (18%) | 87 (32%) | |
| Minimum stent cross-sectional area (mm 2 ) | 8.14 ± 2.99 | 7.25 ± 2.27 | 0.029 |
| Stent expansion (%) | 92.5 ± 25.2 | 82.4 ± 29.4 | 0.044 |
| Postintervention tissue prolapse | 20 (51%) | 94 (35%) | 0.043 |
| Maximum tissue prolapse area (mm 2 ) | 0.41 ± 0.49 | 0.20 ± 0.34 | 0.015 |
| Tissue prolapse volume (mm 3 ) | 1.22 ± 1.65 | 0.43 ± 0.84 | 0.005 |
IVUS findings in the subgroup that underwent PCI using distal protection devices are presented in Table 4 . Of 125 patients treated while using distal protection devices, no reflow was observed in 19 patients (15%). In the 19 patients with no reflow despite use of distal protection devices, lesion site lumen CSA was significantly smaller and plaque CSA, plaque burden, and remodeling index were significantly greater compared to the 106 patients without no reflow. Presence of an intraluminal mass, culprit lesion plaque rupture and multiple plaque ruptures, and positive remodeling were also more common in patients with no reflow. Minimum stent CSA and stent expansion were significantly greater in the no-reflow group. Tissue prolapse after PCI was observed more frequently and maximum tissue prolapse area and tissue prolapse volume were significantly greater in patients with no reflow.



