We assessed the plaque characteristics and postpercutaneous coronary intervention (PCI) outcome according to the remodeling pattern (positive remodeling [PR], n = 113; and intermediate/negative remodeling [IR/NR], n = 198) in 311 saphenous vein graft lesions using intravascular ultrasound. The remodeling index was the ratio of the lesion site saphenous vein graft area to the mean of the proximal and distal references (PR/remodeling index >1.05, IR 0.95 to 1.05, and NR <0.95). Tissue prolapse was defined as tissue extrusion through the stent strut after PCI, and the tissue prolapse volume was calculated by subtracting the lumen volume from the stent volume. The presence of hypoechoic plaque (59% vs 36%, p = 0.001), plaque rupture (26% vs 16%, p = 0.042), multiple plaque rupture (12% vs 5%, p = 0.020), and an intraluminal mass (59% vs 41%, p = 0.002) were more common in the PR lesions than in the IR/NR lesions. The plaque cavity area was significantly greater in the PR lesions than in the IR/NR lesions (0.83 ± 1.43 mm 2 vs 0.42 ± 1.07 mm 2 , p = 0.009). Post-PCI no-reflow (19% vs 9%, p = 0.019) and post-PCI tissue prolapse (53% vs 27%, p <0.001) were observed more frequently, and the tissue prolapse volume was significantly greater after PCI for PR lesions than for IR/NR lesions (0.86 ± 1.30 mm 3 vs 0.34 ± 0.74 mm 3 , p <0.001). PR was the independent predictor of post-PCI no-reflow (odds ratio 2.58, 95% confidence interval 1.25 to 5.64, p = 0.040) and post-PCI tissue prolapse (odds ratio 2.45, 95% confidence interval 1.46 to 5.41, p = 0.045). In conclusion, saphenous vein graft lesions with PR have vulnerable plaque and are associated with no-reflow and tissue prolapse after PCI.
The presence of remodeling within the saphenous vein graft (SVG) is controversial. Nishioka et al showed a lack of remodeling in SVGs; however, this conclusion was disputed by other investigators. Positive remodeling (PR) in native coronary arteries is associated with unstable plaque characteristics, poor postpercutaneous coronary intervention (PCI) outcomes, and poor long-term clinical outcomes compared to intermediate/negative remodeling (IR/NR). However, the clinical findings, plaque characteristics, and post-PCI outcomes according to the remodeling pattern in SVG lesions were not fully assessed. Therefore, the purpose of the present study was to attempt to evaluate the pre- and post-PCI intravascular ultrasound images of SVG lesions and compare the intravascular ultrasound findings between the lesions with PR and those with IR/NR.
Methods
We analyzed the data from the exact same cohort (311 patients who had undergone pre-PCI intravascular ultrasonography, intravascular ultrasound-guided PCI, and post-PCI intravascular ultrasonography for 311 SVG lesions in the Washington Hospital Center) as studied in a recent publication. The institutional review board approved the protocol. The hospital records of all patients were reviewed to obtain clinical demographic data and medical history.
No-reflow was defined as post-PCI Thrombolysis In Myocardial Infarction grade 0, 1, or 2 flow in the absence of mechanical obstruction. Normal reflow was defined as Thrombolysis In Myocardial Infarction grade 3 flow. If Thrombolysis In Myocardial Infarction flow after PCI was 0, 1, or 2 in the absence of angiographic stenosis, repeat intravascular ultrasonography was performed to exclude the possibility of mechanical vessel obstruction. A degenerated SVG was defined as luminal irregularities or ectasia involving >50% of its total length ( Figure 1 ). Thrombus was defined as a discrete, intraluminal filling defect with defined borders, largely separated from the adjacent wall.
Quantitative analysis (CAAS II, Pie Medical, Maastricht, The Netherlands) was performed using standard protocols. With the outer diameter of the contrast-filled catheter as the calibration standard, the reference diameter and minimum lumen diameter were measured in diastolic frames from the orthogonal projections. Perfusion was evaluated according to the Thrombolysis In Myocardial Infarction criteria.
All intravascular ultrasound examinations were performed after intra-SVG administration of 200 μg nitroglycerin using a commercially available intravascular ultrasound system (Boston Scientific/SCIMed, Minneapolis, Minnesota). The intravascular ultrasound catheter was advanced distal to the target lesion, and imaging was performed retrograde to the aorto-ostial junction at an automatic pullback speed of 0.5 mm/s.
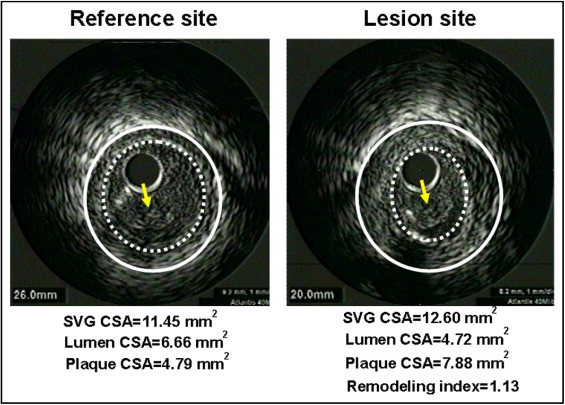
Quantitative and qualitative analyses were performed according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement, and Reporting of Intravascular Ultrasound Studies. Before PCI, we measured the SVG and lumen cross-sectional area using planimetry software (TapeMeasure, Indec Systems, Mountain View, California). The SVG cross-sectional area was measured by tracing the outer border of the entire vein graft. The plaque cross-sectional area was calculated as the SVG cross-sectional area minus the lumen cross-sectional area, and the plaque burden was calculated as the plaque cross-sectional area divided by the SVG cross-sectional area. The lesion site was the image slice with the smallest lumen. If multiple image slices had the same minimum lumen cross-sectional area, the image slice with the largest SVG and plaque cross-sectional areas was measured. The reference images were the single slices with the largest lumen and smallest plaque cross-sectional areas within 10 mm proximally and distally. If the lesion was located at the aorto-ostial site, we used the distal reference only. If the lesion was located at an anastomotic site, we used the proximal reference only. The remodeling index was the ratio of the lesion site SVG cross-sectional area divided by the average of the proximal and distal reference SVG cross-sectional area. PR was defined as a remodeling index >1.05 ( Figure 2 ), IR was defined as a remodeling index between 0.95 and 1.05, and NR was defined as a remodeling index <0.95.
An intraluminal mass had a layered lobulated appearance, evidence of blood flow (microchannels) within the mass, and speckling or scintillation (similar to thrombus in native coronary arteries). A ruptured plaque contained a cavity that communicated with the lumen with an overlying residual fibrous cap fragment. Rupture sites separated by a length of SVG containing smooth lumen contours without cavities were considered to represent different plaque ruptures. A lipid pool-like image was defined as hypoechoic or echolucent material covered with a hyperechoic layer. Hypoechoic plaque was less bright than the adventitia, hyperechoic noncalcified plaque was as bright as, or brighter than, the adventitia without acoustic shadowing, and hyperechoic calcified plaque was brighter than the adventitia with acoustic shadowing. When no dominant plaque composition was found, the plaque was classified as mixed.
At post-PCI IVUS, we measured the minimum stent cross-sectional area. Stent expansion was calculated as the minimum stent cross-sectional area divided by mean reference lumen cross-sectional area. Tissue prolapse was defined as tissue protrusion through the stent strut after the procedure, and the tissue prolapse volume was calculated by subtracting the lumen volume from the stent volume.
The Statistical Package for Social Sciences (SPSS) for Windows, version 15.0 (SPSS, Chicago, Illinois) was used for all analyses. Continuous variables are presented as the mean value ± SD. Comparisons were conducted using Student’s t test or nonparametric Wilcoxon test, if the normality assumption was violated. Discrete variables were presented as percentages and relative frequencies. Comparisons were conducted using chi-square statistics or Fisher’s exact test, as appropriate. Logistic regression analysis was used to identify the independent predictors of post-PCI no-reflow and tissue prolapse. A p value <0.05 was considered statistically significant.
Results
The baseline characteristics are summarized in Table 1 . Patients with PR had more hypercholesterolemia and lower ejection fraction compared to those with IR/NR. A trend was seen toward greater baseline creatine kinase-MB in patients with PR compared to IR/NR. No significant differences were seen in graft age or the use of distal protection devices, bivalirudin, glycoprotein IIb/IIIa inhibitors, and pre-PCI clopidogrel loading between the 2 groups. The PercuSurge Guardwire Plus System (Medtronic AVE, Sunnyvale, California) was used in 80 patients, the Angioguard Filter System (Cordis, Miami, Florida) in 25 patients, and the FilterWire EX (Boston Scientific EPI, Santa Clara, California) in 20 patients.
| Variable | Remodeling | p Value | |
|---|---|---|---|
| Positive (n = 113) | Intermediate/Negative (n = 198) | ||
| Age (years) | 69.1 ± 10.7 | 69.5 ± 10.2 | 0.7 |
| Men | 86 (76%) | 148 (75%) | 0.8 |
| Clinical presentation | 0.12 | ||
| Stable angina pectoris | 24 (21%) | 66 (33%) | |
| Unstable angina pectoris | 71 (63%) | 105 (53%) | |
| Non–ST-segment elevation myocardial infarction | 18 (16%) | 26 (13%) | |
| ST-segment elevation myocardial infarction | 0 (0%) | 1 (1%) | |
| Diabetes mellitus | 45 (40%) | 72 (36%) | 0.5 |
| Hypertension | 81 (72%) | 146 (74%) | 0.7 |
| Smoker | 48 (43%) | 78 (39%) | 0.6 |
| Total cholesterol >220 mg/dl | 95 (84%) | 140 (71%) | 0.008 |
| Family history of coronary disease | 35 (31%) | 60 (30%) | 0.9 |
| Duration after saphenous vein graft surgery (years) | 11.6 ± 6.9 | 10.9 ± 6.5 | 0.4 |
| Ejection fraction (%) | 42.5 ± 9.6 | 53.4 ± 11.0 | 0.037 |
| Distal protection device use | 45 (40%) | 80 (40%) | 0.9 |
| Bivalirudin use | 98 (87%) | 171 (86%) | 0.9 |
| Aspirin use | 112 (99%) | 197 (99%) | 0.7 |
| Clopidogrel use | 111 (98%) | 197 (99%) | 0.3 |
| Glycoprotein IIb/IIIa inhibitor use | 26 (23%) | 44 (22%) | 0.9 |
| Prepercutaneous coronary intervention clopidogrel loading | 51 (45%) | 98 (50%) | 0.5 |
| Prepercutaneous coronary intervention creatine-kinase MB (ng/ml) | 2.10 ± 9.33 | 0.98 ± 1.42 | 0.097 |
| Prepercutaneous coronary intervention cardiac troponin I (ng/ml) | 0.63 ± 3.64 | 0.20 ± 0.69 | 0.11 |
| Postpercutaneous coronary intervention creatine kinase-MB (ng/ml) | 9.16 ± 48.70 | 4.39 ± 8.39 | 0.3 |
| Postpercutaneous coronary intervention cardiac troponin I (ng/ml) | 5.68 ± 38.94 | 1.59 ± 3.31 | 0.15 |
The angiographic findings and procedural results are listed in Table 2 . Patients with PR had more shaft lesions (the combination of proximal, middle, and distal lesions, except for ostial and anastomotic lesions, 82% vs 67%, p = 0.006). In contrast, patients with IR/NR had more aorto-ostial lesions. The stent length was significantly longer in patients with PR than in those with IR/NR. Although no significant differences were found in pre-PCI Thrombolysis In Myocardial Infarction flow grade, the use of direct stenting, adjunct balloon angioplasty, and inflation pressure between 2 groups, post-PCI no-reflow was observed more frequently in patients with PR compared to those with IR/NR (19% vs 10%, p = 0.019). Degenerated SVGs and angiographic thrombus were observed significantly more often in patients with PR than in those with IR/NR.
| Variable | Remodeling | p Value | |
|---|---|---|---|
| Positive (n = 113) | Intermediate/Negative (n = 198) | ||
| Narrowed saphenous vein graft to | 0.7 | ||
| Left anterior descending | 23 (20%) | 42 (21%) | |
| Left circumflex | 40 (35%) | 77 (39%) | |
| Right | 50 (44%) | 79 (40%) | |
| Lesion site | |||
| Ostium | 14 (12%) | 50 (25%) | 0.007 |
| Proximal | 42 (37%) | 58 (29%) | 0.15 |
| Middle | 31 (27%) | 38 (19%) | 0.092 |
| Distal | 20 (18%) | 38 (19%) | 0.7 |
| Anastomosis site | 6 (5%) | 14 (7%) | 0.5 |
| Prepercutaneous coronary intervention Thrombolysis In Myocardial Infarction flow grade | 0.9 | ||
| 0 | 5 (4%) | 11 (6%) | |
| 1 | 1 (1%) | 1 (1%) | |
| 2 | 14 (12%) | 25 (13%) | |
| 3 | 93 (82%) | 161 (81%) | |
| Degenerated saphenous vein graft | 59 (52%) | 71 (36%) | 0.042 |
| Thrombus | 50 (44%) | 58 (29%) | 0.046 |
| Stent type | 0.2 | ||
| Sirolimus-eluting stent | 59 (52%) | 95 (48%) | |
| Paclitaxel-eluting stent | 24 (21%) | 32 (16%) | |
| Bare metal stents | 30 (27%) | 71 (36%) | |
| Stent diameter (mm) | 3.34 ± 0.51 | 3.33 ± 0.53 | 0.8 |
| Stent length (mm) | 22.9 ± 8.4 | 19.8 ± 7.2 | 0.001 |
| Direct stenting | 78 (69%) | 135 (68%) | 0.9 |
| Inflation pressure (atm) | 13.4 ± 3.3 | 13.7 ± 3.0 | 0.5 |
| Adjunct balloon | 47 (42) | 82 (41%) | 1.0 |
| Inflation pressure (atm) | 16.2 ± 3.7 | 16.1 ± 3.5 | 0.8 |
| Postpercutaneous coronary intervention Thrombolysis In Myocardial Infarction flow grade | 0.019 | ||
| 0 | 0 (0%) | 1 (1%) | |
| 1 | 0 (0%) | 2 (1%) | |
| 2 | 21 (19%) | 15 (8%) | |
| 3 | 92 (81%) | 180 (91%) | |
| Reference diameter (mm) | 3.53 ± 0.91 | 3.54 ± 0.81 | 0.9 |
| Preminimum lumen diameter (mm) | 1.52 ± 0.54 | 1.56 ± 0.61 | 0.6 |
| Postminimum lumen diameter (mm) | 2.94 ± 0.54 | 2.92 ± 0.62 | 0.8 |
The intravascular ultrasound results are summarized in Table 3 . The reference segment lumen cross-sectional area was significantly smaller in patients with PR than in those with IR/NR. The lesion site lumen cross-sectional area was significantly smaller, the SVG cross-sectional area, plaque cross-sectional area, and plaque burden were significantly greater, and the lesion length was significantly longer in patients with PR than in those with IR/NR. The presence of an intraluminal mass, culprit lesion plaque rupture and multiple plaque ruptures, and hypoechoic plaque were significantly more common in patients with PR than in those with IR/NR. The plaque cavity cross-sectional area was significantly greater in patients with PR compared to those with IR/NR. Stent expansion was significantly greater in patients with PR than in those with IR/NR. Post-PCI tissue prolapse was more frequently observed and the maximum tissue prolapse area and tissue prolapse volume were significantly greater after PCI for lesions in patients with PR compared to those with IR/NR.



