Intravascular Imaging
Misty Humphries
The ability to assess endovascular interventions by real-time means has historically been limited. Angiography provides only a two-dimensional representation of the cylindrical vessel lumen, and subtle plaque irregularities may be missed. Characterization of arterial plaque when imaged by angiography is frequently limited to identification of calcification. Although Ascher et al have advocated duplex ultrasound for guidance of endovascular procedures, it has not been widely accepted, in part due to the personnel support needed and the limited ability of ultrasound to visualize vessels deep in the pelvis and leg with adequate resolution.1 Computed tomography (CT) and magnetic resonance imaging (MRI) are difficult to apply in the intraoperative realm because of equipment limitations, although further imaging refinements may make this easier in the future. Limitations in these modalities have stimulated interest in imaging within blood vessels.
Intravascular imaging is not a new concept. As early as the 1950s, angioscopy was developed in an attempt to determine intraluminal plaque characteristics. This technology requires complete cessation of blood flow, and the large size of the initial devices limited clinical utility. Newer devices are as small as 0.75 mm in outer diameter and can image approximately 1 to 5 mm in depth. They are also mounted with a proximal occlusion balloon to stop blood flow and permit clear imaging. Most of these smaller devices are still in development, and clinical use has been limited. Over the same time frame, intravascular ultrasound (IVUS) has been steadily refined. Not until the late 1980s were Yock et al able to reduce the size of a single ultrasound transducer system in order to image coronary arteries, making IVUS a clinically useful tool.2 Since then, IVUS systems have undergone multiple refinements to make them the small easily applied imaging systems we have today.
Optical coherence tomography (OCT), a newer technology, is rapidly gaining momentum and is being incorporated into peripheral devices to improve endovascular capabilities. It uses near infrared light as opposed to ultrasound waves. This allows OCT devices to have a markedly improved resolution compared to IVUS, the trade-off being limited to evaluating vessels of relatively small diameter. Device improvement and development have allowed physicians who deliver vascular care to better understand intravascular pathology and the pitfalls of interventions.
VASCULAR WALL ANATOMY AND ATHEROSCLEROSIS
A complete discussion of arterial wall anatomy is beyond the scope of this chapter, but a simple understanding of basic anatomy and plaque characteristics warrants mentioning, given that the greatest advantage of intravascular imaging is the ability to define plaque composition and identify features that may affect procedural outcomes. The arterial wall is composed of three distinct layers: the tunica intima, media, and adventitia. The intima layer is closest to the flowing blood and consists of endothelial cells separated from the subendothelial space by the basal lamina. The subendothelial space is an extracellular matrix composed of proteoglycans and collagen that extends to the internal elastic lamina.
Atherosclerotic lesions develop as the endothelium increases expression of leukocyte adhesion molecules. A subsequent increase in endothelial permeability allows leukocytes and lipoproteins to move into the subendothelial space where macrophages and smooth muscle cells are located. With progression of the lesion, lipoproteins are taken up by macrophages and transformed into foam cells that form the lipid core of atherosclerotic lesions. Progressive inflammation in this area also stimulates smooth muscle cell proliferation in the subendothelial space. Activated smooth muscle cells continue the production of growth factors and stimulate further proliferation. As collagen and extracellular matrix are deposited, the fibrous cap becomes more developed. With increased levels of inflammatory mediators within the vessel wall, both smooth muscle cells and endothelial cells may undergo apoptosis causing thinning of the cap. This can lead to vulnerable or unstable plaque. With rupture of this plaque, the inner lipid core becomes exposed along with other prothrombotic elements and subsequent thrombosis may occur.3 The ability to detect these histologic changes and treat lesions with targeted therapy based on plaque characteristics has been a long-term goal. IVUS offers a tool to improve outcomes and refine new therapeutic intravascular technology.
INTRAVASCULAR ULTRASOUND
Technical Considerations
There are two varieties of IVUS catheters: mechanical and phased array (Fig. 31.1). Mechanical transducers use electrical current generated through a piezoelectric crystal to produce and receive sound waves. Typically, mechanical transducers have a single transducer element that rotates circumferentially on a drive shaft at approximately 1900 rotations per minute to create a cross-sectional image. Phased array transducers are stationary. They have multiple transducer elements, typically 64 in current catheters, positioned in a radial fashion that fire in circumferential succession. This produces an array of
images, which are processed into a 360-degree cross-sectional image of the vessel lumen and wall.
images, which are processed into a 360-degree cross-sectional image of the vessel lumen and wall.
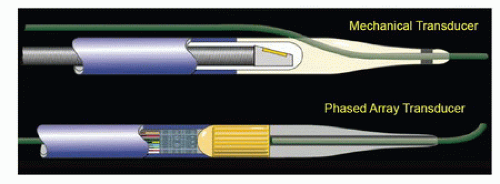 FIGURE 31.1. Mechanical and phased array transducers. Mechanical transducer images may have artifact related to the wire position. (Courtesy of Boston Scientific Corporation, Natick, MA.) |
Both types of transducers have limitations and generate artifacts that the user must be familiar with. Mechanical transducers typically have a wire channel that runs alongside the transducer that generates a wire artifact in the images (Fig. 31.2). In addition, mechanical transducers can trap small bubbles that result in air speckling if not flushed properly. Nonuniform rotational distortion (NURD) is a rotational distortion that occurs with mechanical transducers. This is typically seen in tortuous vessels, with kinking of guide catheters, or when hemostatic Tuohy-Borst valves are tightened excessively over the catheter. Excess stress on the IVUS catheter causes bending, and the rotational speed of the transducer varies, causing image acquisition to be distorted. Manufacturers of mechanical transducers have introduced corrective software to prevent this distortion. Phased array transducers can have ring-down artifacts. This represents an area immediately surrounding the catheter obscured by acoustic oscillations within the transducer. Adjustments in the gain of the transducer can be made once the catheter is outside of the sheath or guide catheter and not apposed to the arterial wall to minimize this artifact. Care must be taken to avoid adjusting too much, as true luminal signals can also be suppressed.4
IVUS catheters range in frequency from 8 to 50 MHz. As with all ultrasound probes, higher frequencies provide greater spatial resolution. The trade-off for using higher frequency probes is decreased depth of tissue penetration. Balancing these two factors is imperative when selecting the appropriate catheter for intravascular imaging. Catheters suitable for imaging the aorta and inferior vena cava (IVC) have a frequency range from 9 to 15 MHz. These catheters are typically able to image vessels with a maximum diameter of 5 to 6 cm, depending on the system. Optimal imaging of the iliac and common femoral arteries and veins can be obtained with catheters of 15 to 20 MHz. Smaller vessels, such as the superficial femoral, tibial, and renal arteries, are best imaged with transducers of 20 to 40 MHz (Table 31.1). Although vessel size may initially dictate the catheter used, other factors such as delivery system, sheath size, and wire access also factor into the selection of the appropriate catheter for the case at hand.
TABLE 31.1 CHARACTERISTICS OF CURRENT COMMERCIALLY AVAILABLE PHASED ARRAY AND MECHANICAL IVUS CATHETERS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
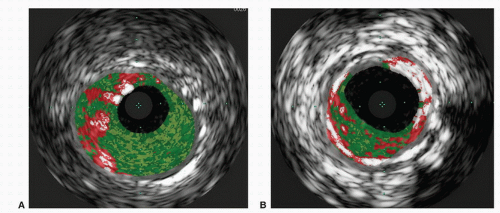 FIGURE 31.3. Virtual histology of peripheral plaque. A, Fibro-fatty plaque with small areas of necrotic core. B, Fibroatheroma with dense areas of calcification. |
Interpretation of IVUS imaging can be aided by two specific technologies: virtual histology and ChromaFlo®. Virtual histology is a computer-generated color map created from the reflected ultrasound signals. This map is displayed on the screen superimposed over the gray-scale image. Each of the four colors used represents a different component of plaque (Fig. 31.3). Deep green represents fibrous plaque, lighter green is fibro-fatty plaque, white represents dense calcium, and red is necrotic core. In an attempt to better understand the ability of virtual histology to characterize plaque, the Virtual Histology Intravascular Ultrasound Assessment of Carotid Artery Disease: The Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study used virtual histology to assess carotid plaque prior to endarterectomy in 15 patients. Histologic composition of the plaques was compared to the virtual histology generated by IVUS. The authors found the diagnostic accuracy of virtual histology to range from 72.4% for calcified fibroatheroma to 99.4% for thin-cap fibroatheroma. The specificity ranged from 82% for intimal thickening to 100% for thincap fibroatheroma.
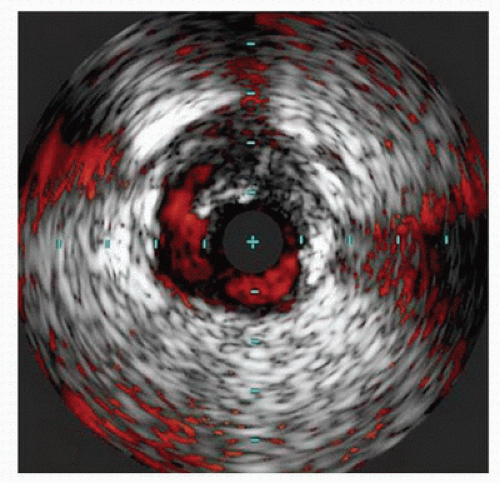 FIGURE 31.4. ChromaFlo® imaging showing delineation of an edge dissection after angioplasty. (Images courtesy of Volcano Corporation, San Diego, CA.) |
Small and mid-sized vessel phased array catheters are also able to offer color flow (ChromaFlo®) technology. When the location or patency of the vessel lumen is unclear, color flow imaging can be turned on to display the flow channel of the vessel (Fig. 31.4). Applications of this technology include evaluating the luminal irregularity of plaque and stent apposition. This technology has also been mounted on a reentry needle for use in sub-intimal angioplasty.
Applications
Venous
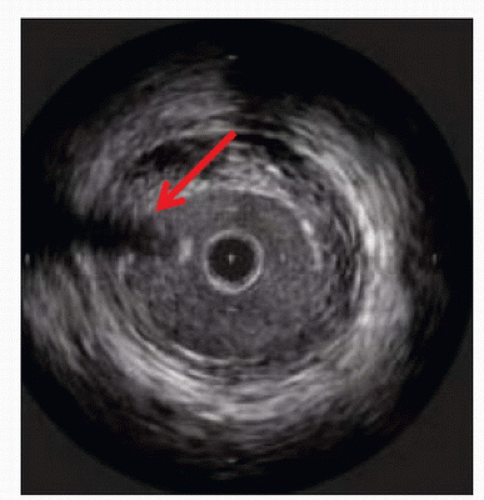
IVUS has emerged as an important adjunct for endovascular venous procedures. When used during thrombolytic procedures, IVUS can identify residual thrombus. Acute thrombus is difficult to visualize completely with IVUS images (Fig. 31.5). Chronic thrombus, on the other hand, is usually devoid of red blood cells and contains a higher concentration of fibrin, making it more echogenic and easier to distinguish from the vessel wall. Bands, frozen valves, spurs, or venous webs as the cause of thrombosis may not be easily identified with venography. IVUS can delineate irregularities that may be amenable to simple angioplasty and can determine if stent placement is required.
Use of IVUS has become essential in the treatment of iliac vein obstruction or stenosis resulting from arterial compression, also known as May-Thurner syndrome. Classic May-Thurner is caused by compression of the left common iliac vein by the right common iliac artery. Two additional areas of possible compression are located where the right external iliac artery crosses the right external iliac vein and where the left hypogastric artery crosses the left external iliac vein. Venography may show a flattening of the vein where compressed, and chronic compression can lead to scarring and thrombosis (Fig. 31.6). Venography can also severely
underestimate the degree of venous stenosis, and stent sizing after recanalization without IVUS can lead to significant undersizing. Therefore, IVUS has become the preferred method for identifying the area of greatest venous narrowing to ensure accurate stent placement.
underestimate the degree of venous stenosis, and stent sizing after recanalization without IVUS can lead to significant undersizing. Therefore, IVUS has become the preferred method for identifying the area of greatest venous narrowing to ensure accurate stent placement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree