4 Intraoperative Transesophageal Echocardiography
Key Points
• There is a vast spectrum of abnormalities in patients with congenital heart disease (CHD), and there are usually several appropriate surgical options for repair. The echocardiographer must have an excellent understanding of the surgical repair to interpret intraoperative findings.
• The intraoperative presurgical transesophageal echocardiography (TEE) is used to confirm the diagnosis and help guide the hemodynamic management before cardiopulmonary bypass (CPB), if needed. Because of the unique views and excellent resolution of TEE, new abnormalities are occasionally diagnosed prerepair that call for a revision of the surgical plan.
• With the less than ideal conditions of the operating room with respect to lighting, time constraints, and labile hemodynamic conditions, examinations are more directed and less detailed than a comprehensive transthoracic study.
• The hemodynamic condition of the patient under anesthesia may differ from baseline and may alter findings. For example, the severity of valve regurgitation may be less under anesthesia. The decision to repair or replace a valve is best made based on preoperative imaging. At the conclusion of a bypass, satisfactory hemodynamic conditions should be present for assessment of TEE.
• Postprocedure TEE is used to evaluate the repair and to guide postbypass hemodynamic management. There are circumstances when residual abnormalities are left because of surgical limitations or patient considerations. For example, these compromises are frequently made in the case of valvular heart disease in very young patients.
• TEE has significant limitations in the direct assessment of the aortic arch and descending aorta because the trachea interferes with adequate imaging. Doppler techniques may demonstrate indirect evidence of flow but are often suboptimal. Consequently, TEE is rarely used in the repair of isolated coarctations or interruptions of the aorta.
Practical Considerations
• Ensure an adequate level of anesthesia before probe placement, especially in patients at risk of complications from sympathetic stimulation, e.g., patients with tetralogy of Fallot (TOF), other forms of dynamic outflow tract obstruction, pulmonary hypertension (PHTN).
• The TEE probe may cause hemodynamic or respiratory changes in young patients because of its proximity to the left atrium (LA), pulmonary veins (PVs), and airway. When there is doubt about the cause of a serious hemodynamic or airway problem, it is best to remove the probe.
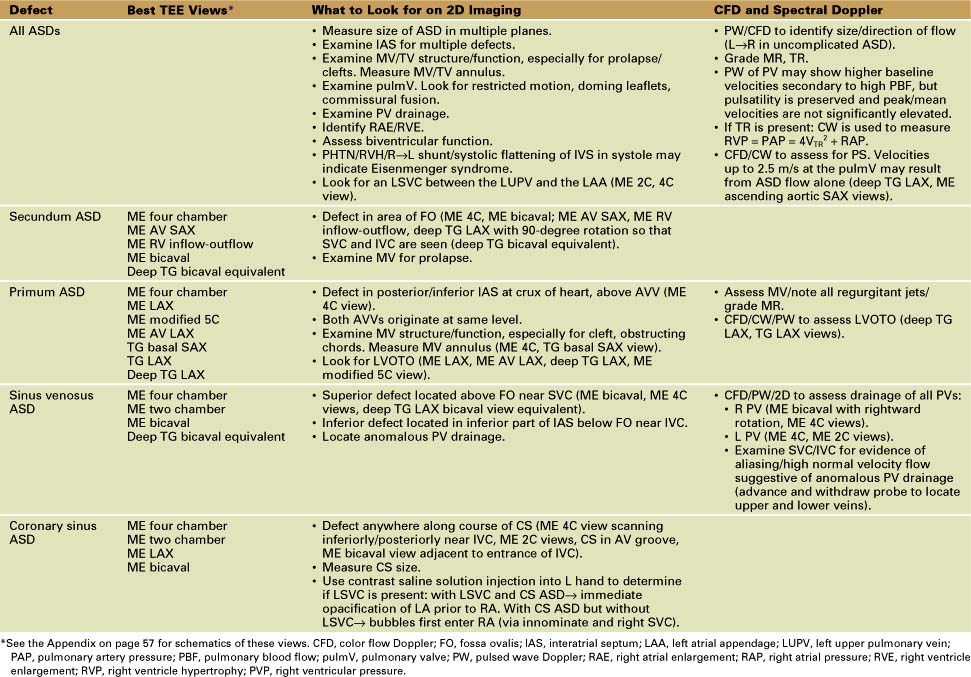
Atrial Septal Defects (Table 4-1)
Key Points
Secundum Atrial Septal Defects (Fig. 4-1)
• If left untreated, Eisenmenger syndrome develops in about 5% (irreversible pulmonary vascular disease).
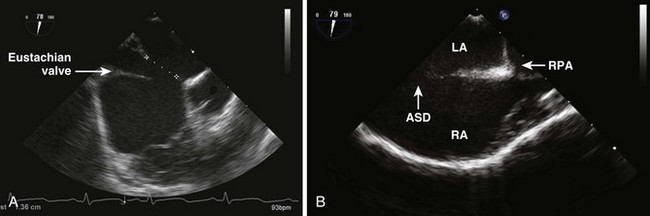
Primum Atrial Septal Defects (also called Partial Atrioventricular [AV] Canal, Defect)
• Left ventricular outflow tract obstruction (LVOTO) can develop from:
• Long tunnel-like obstruction of the left ventricular outflow tract (LVOT) (“goose-neck” deformity).
Sinus Venosus Atrial Septal Defects
• Sinus venosus ASDs are associated with PAPVD, especially right PVs that drain into the superior vena cava (SVC) or right atrium (RA).
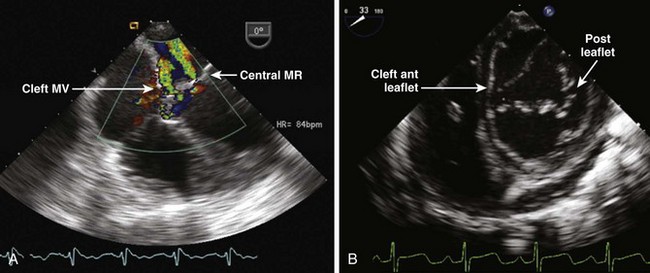
Coronary Sinus Atrial Septal Defects (also called Unroofed Coronary Sinus)
• Results in shunting between the LA and the RA via the missing “roof” of the CS. The amount of unroofing is variable (defect may be partial or complete) (Fig. 4-3).
• The CS may appear large even without an LSVC because of flow from the shunt. It is crucial to identify the presence of an LSVC because it alters the surgical repair.
Principles of Surgical Management
• In the pediatric age group, it is very rare to have to perform a concomitant tricuspid valve (TV) repair, but it may be necessary in older patients with moderate to severe TR.
Sinus Venosus Sinus Atrial Septal Defects
• The SVC-RA junction may be augmented with a pericardial patch to avoid SVC obstruction: two-patch technique may be necessary, one to baffle the PV and one to augment the SVC.
• When PVs drain into the high SVC or there is difficulty making an unobstructed baffle, a Warden procedure may need to be performed in which the SVC is disconnected and reattached to the right atrial appendage. The PV “stump” (the cardiac end of the SVC containing the anomalous PV) is baffle closed to the LA.
Postoperative TEE Assessment
• Assess MV function. After a primum repair, MR can be eccentric and composed of multiple jets. Grading can be difficult. Use the vena contracta width (<3–5 mm is ideal), density of spectral jet, duration of regurgitation, area/pattern of jet (wrap around ventricle), and effect on PV flow pattern. Less than mild MR is best for a durable repair, but moderate MR may be acceptable if further repair results in mitral inflow obstruction.
• Sinus venosus ASD: Confirm appropriate drainage of PVs into the LA without narrowing of PV, SVC, SVC baffle, or SVC-RA appendage anastomosis. SVC and PV flow should be laminar, low velocity, and phasic.
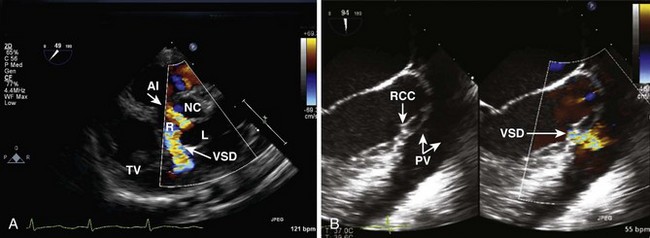
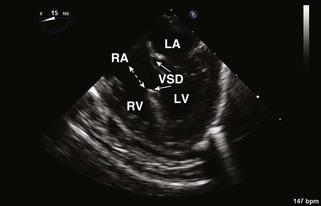
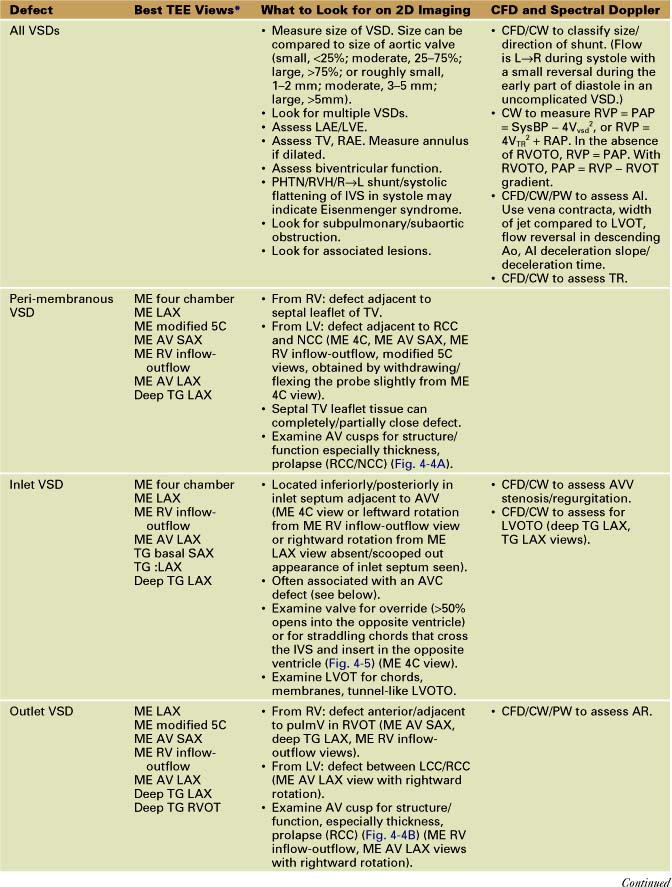
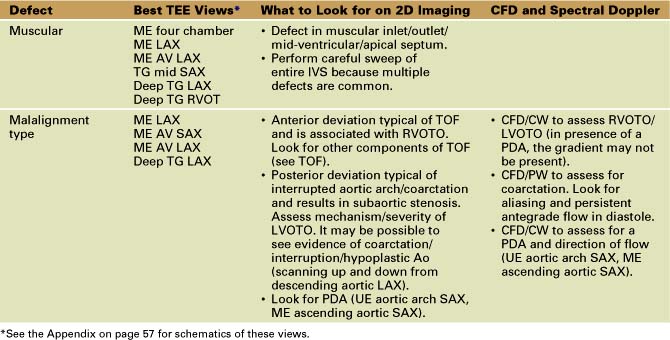
Ventricular Septal Defects (Table 4-2)
• Outlet (also called subpulmonary, supracristal, doubly committed, subarterial, infundibular, conal)
Key Points
Principles of Surgical Management
• In the presence of a large VSD, PHTN, evidence of Eisenmenger syndrome, or R→L flow through the VSD, cardiac catheterization may be required to assess pulmonary vascular resistance (PVR) before closure of a VSD.
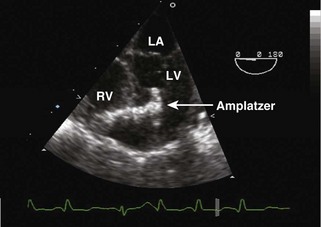
• At this time, devices are not yet approved for routine closure of VSDs, although devices for closure of muscular/perimembranous defects are available. A hybrid approach with catheter access through a right ventriculotomy with TEE guidance is useful in some cases (Fig. 4-6).
• Surgical closure is with a synthetic patch via RA/RV/infundibular (transpulmonary)/transaortic/rarely LV approach.
• Surgical management in patients with subaortic obstruction from a posterior malaligned VSD is complex and may involve LVOT enlargement (Konno procedure) or may have to follow a single ventricle (SV) pathway.
Postoperative TEE Assessment
• Verify VSD closure and look for residual/additional VSDs. Residual acceptable VSD flow should be high velocity (restrictive). About 40% of patients have a residual VSD on intraoperative TEE. Most are small or moderate. Long-term follow-up shows that the majority close. Additional parameters to quantitate size are Qp/Qs (pulmonary/systemic blood flow) determined by intraoperative blood gas analysis (<1.5, small; 1.5–2, moderate; >2, large) or right ventricular pressure/left ventricular pressure (RVP/LVP) <0.5, small; 0.5–0.75, moderate; >0.75, large).
• Examine TV/AV. Look for prolapse/perforation of a cusp or poor resuspension of the TV around annulus. Confirm that there is no significant TR or AR.
Atrioventricular Canal Defects (also called Atrioventricular Septal Defects or Endocardial Cushion Defects) (Table 4-3)
TABLE 4-3 BASIC ECHO PRINCIPLES WHEN IMAGING AVC DEFECTS
| Best TEE Views* | What to Look for on 2D Imaging | CFD and Spectral Doppler |
|---|---|---|
• Examine the configuration of the AVV. Look for straddling/aberrant chords/AVV override, MV cleft, leaflet prolapse (ME 4C, ME LAX, TG basal SAX deep TG LAX views). • Evaluate size/function of ventricles and whether the defect is balanced (ME 4C, TG mid-SAX views). |
* See the Appendix on page 57 for schematics of these views.
Complete AVC
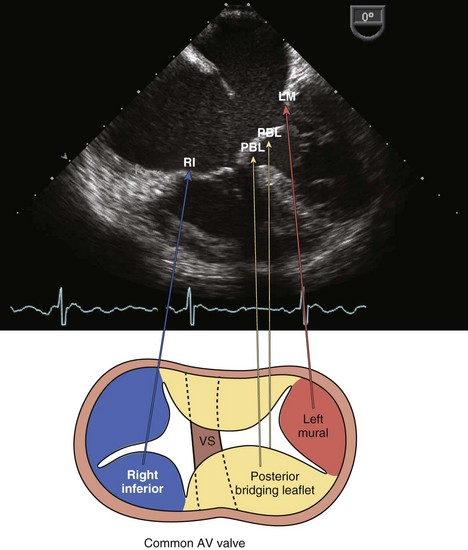
• Single AVV with multiple leaflets, usually an anterior (superior) and posterior (inferior) leaflet that bridges the IVS and several lateral leaflets (Fig. 4-7).
• Straddling chords that insert in the opposite ventricle may prohibit the creation of two competent AVVs and necessitate a SV repair.
• Often the RV appears small compared with the LV because of the large VSD. Concerning is a small LV (unbalanced AVC), which may indicate the need for a SV repair.
Principles of Surgical Management
• A one- or two-patch technique or the so-called Australian technique (stitch closure of VSD with patch closure of ASD) is used to close the ASDs/VSDs and bridge the anterior/posterior leaflets.
• The atrial patch is usually placed so that the CS drains into the LA. An LSVC draining to the CS would preclude that.
• Residual MV cleft is usually closed unless significant valve distortion would result in mitral stenosis (MS)/MR.
Tetralogy of Fallot (Table 4-4)
TABLE 4-4 BASIC ECHO PRINCIPLES WHEN IMAGING TOF
| Best TEE Views* | What to Look for on 2D Imaging | CFD and Spectral Doppler |
|---|---|---|
• Assess VSD location(s)/size (ME AV LAX, ME LAX, withdraw from ME 4C, ME RV inflow-outflow, ME AV SAX, deep TG LAX views). | ||
• Assess morphology/function of pulmV. Look for doming leaflets with commissural fusion. Measure annulus Z score. Z score < −2.5 to −3 generally warrant transannular patch. |
* See the Appendix on page 57 for schematics of these views.
Key Points
All Forms of Tetralogy of Fallot
• Consist of RVOTO, an anterior malaligned VSD, RVH, and an overriding aorta. The VSD is usually large and unrestrictive. Associated defects include PFO, ASD, and additional VSDs.
• RVOTO is secondary to a combination of obstruction at multiple levels: subpulmonary/infundibular narrowing/pulmonary valve annular hypoplasia/PS/main pulmonary artery (PA) or branch PA hypoplasia. RV hypertrophy results from obstruction and worsens over time. Rarely, there is intracavitary obstruction similar to a double-chamber RV.
• There is a 25% incidence of a right aortic arch (the arch goes over the right bronchus, but this is not seen by TEE). However, abnormalities in the course of the descending aorta should increase suspicion because although a left arch can exist with a right descending aorta, it is unusual.
• Coronary artery anomalies are common. In about 3%, the left anterior descending artery comes from the right coronary artery (RCA) and crosses the RVOTO.
Tetralogy of Fallot with Pulmonary Stenosis
• The most common location of PA hypoplasia is the left PA at the insertion point of the ductus. The left PA is not well seen by TEE.
Tetralogy of Fallot with Pulmonary Atresia
• Variable degrees of hypoplasia of the main or branch PAs. There can be nonconfluent PAs, PAs supplied by a ductus with or without multiple aortopulmonary collateral arteries, and absent PAs. In the latter case, pulmonary blood flow is supplied by multiple aortopulmonary collateral arteries.
Tetralogy of Fallot with an Atrioventricular Canal
• Components of both diseases present, but RVOTO results in less PBF and a smaller common AVV orifice than with an isolated AVC, making the repair more difficult.
Tetralogy of Fallot with an Absent Pulmonary Valve
• Pulmonary annulus is hypoplastic with no pulmonary valve leaflets, resulting in severe pulmonary valve insufficiency (PI). PAs are large/aneurysmal. Often there is severe RV dilatation. The degree of airway compromise is the overriding determinant of prognosis.
Principles of Surgical Management (Table 4-5)
TABLE 4-5 PRINCIPLES OF SURGICAL MANAGEMENT
| Defect | Most Common Surgical Apapproach |
|---|---|
| All TOF (except TOF-PA) | • Complete repair consists of VSD closure/division/limited resection of RV muscle bundles/pulmonary valvotomy and commissurotomy/transannular patch/PA plasty if indicated. RA/RV approach for VSD, and may need separate infundibular incision for obstructing outlet components. |
| TOF-PS | |
| TOF-PA | |
| TOF-AVC | |
| TOF-APV | |
| DORV | • The spectrum of repairs varies from a TOF-like repair to an arterial switch procedure and occasionally other options. |
Postoperative TEE Assessment
• Immediately post-bypass, there may be acute worsening of right ventricular diastolic and systolic function secondary to multiple factors such as bypass and ischemic cross-clamp times, a ventriculotomy, or, rarely, coronary injury. Additional volume and pressure loads on the RV from PI, AVV regurgitation, residual VSD, or RVOTO can create an intolerable hemodynamic burden. The postoperative course is especially difficult after TOF with AVC if the repair is suboptimal. Interpretation of post-CPB TEE must take these facts into account.
• Visualize the VSD patch and look for a residual VSD. Reexamine the IVS because lower right ventricular pressures may unmask previously unrecognized VSDs.
• Locate/measure residual RVOTO. Obstruction with RVP more than two thirds systemic requires revision. The exception is myocardial hypertrophy causing dynamic obstruction, which generally resolves with time and/or altered hemodynamic management (volume loading, decreasing inotropic support, etc.).
• Examine PFO/atrial shunt and note direction of flow. R→L flow provides a crude indication of right ventricular diastolic dysfunction and provides an explanation for systemic desaturation.
Transposition of the Great Arteries (Table 4-6)
TABLE 4-6 BASIC ECHOCARDIOGRAPHIC PRINCIPLES WHEN IMAGING d-TGA