Internal Carotid Artery
Peter Lanzer
Ralf Weser
Carotid Artery Disease and Stroke
Stroke, the destruction of brain tissue, may be related to focal or global ischemia (approximately 80%) or hemorrhage (approximately 20%). Ischemic strokes are caused mostly by focal arterial occlusions due to thrombosis or embolization, and less frequently by global hypoperfusion or coagulation disorders; hemorrhagic strokes are due to intracerebral hemorrhage (rupture of small intracerebral vessels) or subarachnoid hemorrhage (rupture of arterial aneurysms).
The majority of ischemic strokes are due to atherothrombotic and thromboembolic extracranial carotid artery disease; other less frequent vascular pathologies include spontaneous dissections, arteritides, fibromuscular dysplasia, arterial kinking, aneurysms, and complications of head and neck neoplasias. In small intracranial vessels, other vasculopathies may be also responsible such as noninflammatory degenerative disorders, Moyamoya disease, and persistent vasospasms.
Embolic occlusions are mostly thrombogenic, related to known cardiac sources including the left atrium in patients with atrial fibrillation or left atrial myxoma, the left ventricle in patients with acute myocardial infarction or severe left ventricular dysfunction, and aortic or mitral valves in patients with endocarditis or degenerative valvular disorders. Other potential sources of thrombogenic emboli include deep peripheral veins in patients with patent foramen ovale or interventricular septum defects, and ascending aortic atherothrombotic disease in patients with advanced generalized atherosclerosis. Nonthrombogenic emboli such as atherosclerotic debris, air, nitrogen bubbles, fat emboli, septic emboli, or tumor tissue are less frequent and mostly associated with specific situations such as cardiac surgery, major trauma, and cases of sudden decompression (e.g., Caisson’s disease).
Global brain hypoperfusion is related to states associated with major circulatory compromise such as cardiac arrest, cardiogenic shock, or severe arrhythmias (for a review of stroke etiologies, see references 1 and 2). Furthermore, a number of genetic loci associated with stroke have been identified (for review, see reference 3).
In a recent study conducted by the American Stroke Association and American Heart Association in the United States in 2002, of 700,000 strokes, of which 500,000 were new and 200,000 recurrent; 88% were ischemic and 12% were hemorrhagic (9% intracerebral and 3% subarachnoid hemorrhages). The age-adjusted stroke incidence rates per 100,000 for the first stroke were 167 for white male patients, 138 for white female patients, 323 for black male patients, and 260 for black female patients.4
Major nonmodifiable risk factors for stroke include age, gender, race, ethnicity, and heredity; major potentially modifiable risk factors for ischemic stroke include hypertension, heart disease (mainly atrial fibrillation, left ventricular dysfunction, and valvular diseases), diabetes mellitus, lipoprotein disorders, smoking, alcohol, and drug abuse, adverse lifestyle habits, contraceptives, and—importantly—asymptomatic carotid artery disease and transitory ischemic attack. Major risk factors of intracerebral hemorrhage include hypertension, smoking, and heavy alcohol abuse (for review, see reference 5).
Carotid artery disease was recognized as a cause of stroke during the second half of the 19th century by a number of eminent pathologists and clinicians; however, it appears that in clinical medicine “the prevailing notion held by most physicians was that strokes were caused by intracranial vascular disease” lasted well into the 20th century (for review, see reference 6). Among those who provided major contributions to understanding the extracranial pathogenesis of stroke, one
should perhaps at least mention the name of J. Ramsay Hunt (1872–1937), who clearly established the link between ipsilateral partial or total occlusion of a carotid artery and contralateral clinical symptoms.7 In a more recent era, C. Miller Fisher (b. 1913) is credited with providing a major contribution to our present understanding of the principal role of the atherosclerotic carotid artery disease in the pathogenesis of stroke (for review, see reference 8).
should perhaps at least mention the name of J. Ramsay Hunt (1872–1937), who clearly established the link between ipsilateral partial or total occlusion of a carotid artery and contralateral clinical symptoms.7 In a more recent era, C. Miller Fisher (b. 1913) is credited with providing a major contribution to our present understanding of the principal role of the atherosclerotic carotid artery disease in the pathogenesis of stroke (for review, see reference 8).
Besides the traditional and new risk factors for stroke, local factors such as unsteady flow conditions at the carotid flow divider9 associated with low shear stress10 and cyclic mechanical strain11,12 are important factors in the localization, development, and progression of extracranial carotid atherosclerosis.
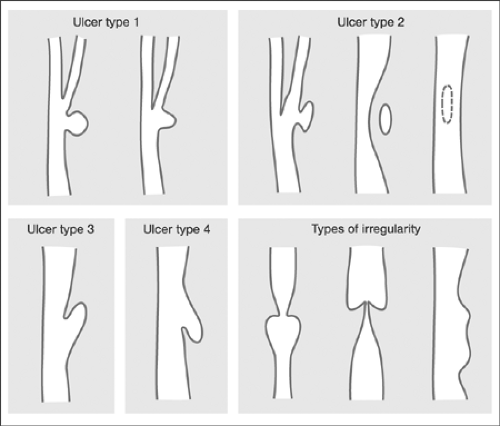
The pathogenetic and clinical significance of carotid artery disease is based on its propensity to reduce the blood flow locally because of atherothrombosis, or peripherally because of embolization. Whereas in the early days, the severity of the local disease was considered the most critical factor in stroke etiology, the importance of the embolization potential of internal carotid lesions in addition to their severity has increasingly been recognized. Thus, on the basis of ultrasound with integrated backscatter, echolucent atherosclerotic plaques with low integrated backscatter can be visualized as vulnerable, lipid-and macrophage-rich carotid plaques.13,14 The embolization potential of plaque ulcerations has also been determined.15 More recently, the irregularity of the plaque surface seen on angiography has been recognized as a predictive risk factor for ipsilateral ischemic stroke in all degrees of stenosis; the predictive power increases with increasing severity of stenosis.16 However, while the angiographic severity of stenosis can be measured, and criteria for making measurements comparable have been established,17,18 morphologic criteria are more difficult to identify objectively. In fact, the degree of concurrence between carotid angiography and surgical observation in detecting carotid plaque ulcerations has been found to be low (Table 7-1) (for review, see reference 19). Nevertheless, a strong association between histology and angiographic carotid artery surface morphology has been reported in a recent study. Here, four different morphologies of carotid artery ulcerations (ulcer types 1 to 4) and two different types of carotid artery surface irregularities (a, b) are described as distinct markers of plaque instability Fig. 7-1.20
TABLE 7-1. Studies Comparing Angiographic Carotid Plaque Surface Morphology with Pathology | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Total or subtotal occlusions of the internal carotid artery or occlusions of its branches may remain clinically silent, or they may produce specific and usually sudden clinical neurological syndromes that are characteristic of the site of the brain ischemia or infarction. In approximately 50% of cases, stroke may be preceded by transient ischemic attacks. In patients with carotid artery disease, middle cerebral artery syndromes are characterized by motoric and sensory impairments of the contralateral face, arm, and leg, vision disturbances, usually homonymous hemianopsia or homonymous quadrantonopsia, or paralysis of conjugate gaze to the opposite side (patient looks toward the
infarcted side). If the lesion is on the dominant side of the brain, aphasia may be present; conversely, if the nondominant side is involved, unilateral neglect and agnosia or anterior cerebral artery syndromes associated with motoric and sensory impairment of the contralateral lower extremity with impaired gait and stance might be present. In addition to the hemispheric syndromes (rarely bilateral syndromes), monocular visual disturbances due to impaired blood flow through the ophthalmic artery may be present. Ischemic syndromes of the posterior cerebral circulation secondary to carotid artery disease are rare (for a review of neurological syndromes associated with carotid artery disease, see reference 2). Interventionists performing carotid artery procedures must be thoroughly familiar with the major neurological manifestations potentially associated with thromboembolic complications of extracranial carotid disease in peri-interventional settings.
infarcted side). If the lesion is on the dominant side of the brain, aphasia may be present; conversely, if the nondominant side is involved, unilateral neglect and agnosia or anterior cerebral artery syndromes associated with motoric and sensory impairment of the contralateral lower extremity with impaired gait and stance might be present. In addition to the hemispheric syndromes (rarely bilateral syndromes), monocular visual disturbances due to impaired blood flow through the ophthalmic artery may be present. Ischemic syndromes of the posterior cerebral circulation secondary to carotid artery disease are rare (for a review of neurological syndromes associated with carotid artery disease, see reference 2). Interventionists performing carotid artery procedures must be thoroughly familiar with the major neurological manifestations potentially associated with thromboembolic complications of extracranial carotid disease in peri-interventional settings.
Diagnostic Evaluations
In addition to the patient’s clinical history and a physical examination, the diagnosis of carotid artery disease should also be based on direct visualization of the carotid arteries using one of the available imaging modalities. Carotid artery ultrasound, magnetic resonance (MR) and computed tomography (CT) angiography may be used for primary diagnostic purposes; however, in patients considered for revascularization and in peri-interventional settings only conventional and digital subtraction angiography, DSA, are applicable.
Duplex ultrasound (Doppler flow measurement plus B-mode image) allows functional and morphologic assessment of the carotid artery stenosis on the basis of measurements of the peak systolic and end-diastolic velocity, spectral dispersion, and the carotid index (defined as a ratio of peak systolic velocities of the poststenotic internal carotid artery and common carotid artery),21 and visual assessment of the entire common carotid and, to a certain extent, the extracranial segments of the internal and external carotid arteries. The opportunity to define potentially unstable echolucent (as opposed to echogenic) atherosclerotic plaques via B-mode ultrasound is of particular clinical interest.13,22 Importantly, a carotid index of >4.0 has been shown to be a highly accurate predictor of high-grade (70% to 99%) stenosis according to the North American Carotid Endarterectomy Trial (NASCET) criteria.23 The limitations of duplex ultrasound include high operator dependence, low sensitivity in low-grade lesions, and a tendency to overestimate severity (for review, see reference 24). Transcranial Doppler using transtemporal, orbital, transforamental, and submandibular windows may be used in conjunction with duplex ultrasound to evaluate the impact of the extracranial artery disease on intracranial hemodynamics, including assessment of collateral flow in the circle of Willis, reversal of flow in the ophthalmic and anterior cerebral arteries, absence of ophthalmic or carotid siphon flow, and reduced middle cerebral artery flow velocity and pulsatility.25 In addition, transcranial Doppler is useful in a more comprehensive evaluation of pathophysiology of cerebrovascular disease, including the detection of microemboli (for review, see reference 26). Three-dimensional and compound ultrasound imaging techniques might further improve the definition of carotid artery atherosclerotic plaque morphology, and clinical utility of MR angiography and CT angiography in the assessment of carotid artery disease in conjunction with neuroimaging using MR and CT are summarized in references 24 and 27.
Despite its numerous shortcomings, x-ray carotid artery angiography, which was introduced in 192728 and became popular after the percutaneous technique of establishing arterial access,29 dedicated catheterization instrumentation,30 and digital imaging technology31 had become available, continues to represents the gold standard in neurovascular imaging and is the only angiographic technique available as a guide during interventional therapy on the carotid artery.
Neuroangiography is used to evaluate intracranial and extracranial head and neck circulation following selective catheterization of the target arteries. The recommended indications for neuroangiography are summarized in Table 7-2.32 Technique and interpretation of diagnostic neuroangiography has been extensively reviewed in the literature.33,34,35
To evaluate patients for extracranial carotid artery revascularization, standard imaging protocols are implemented that include angiography of the aortic arch and the main thoracocervical vessels and selective bilateral extracranial and intracranial carotid angiograms in at least two, preferably orthogonal, projections. In patients with complex cerebrovascular anatomy and multiple collateral pathways, all four neck arteries, carotids and vertebrals, must be selectively visualized to fully assess the intracranial blood supply.
To evaluate patients for carotid artery stenting (CAS), a suitable femoral artery access must be available, and the technical ability to reach and to cross the target vessel must be ensured. To determine whether the target internal carotid artery lesion is accessible using either one of the common femoral arteries access sites, the course and the morphology of the ipsilateral iliac artery, descending aorta, aortic arch, brachiocephalic trunk, and the common carotid arteries should be evaluated.
TABLE 7-2. Indications for Diagnostic Neuroangiography | |||
|---|---|---|---|
|
Specifically, the aortic arch must be assessed to define its form and elongation and the angle of the take-off of the target common carotid artery. Particularly in patients with elevated diaphragm and arterial elongation, the entire aortic arch may be shifted upward, usually displacing the ascending limb of the thoracic aorta laterally and thereby often creating a steep up-sloping shoulder. This aortic arch configuration is indicative of greater technical difficulty in approaching the target vessel. In addition, the presence of associated disease of the aortic arch and of the nontarget cerebrovascular vessels should be noted.
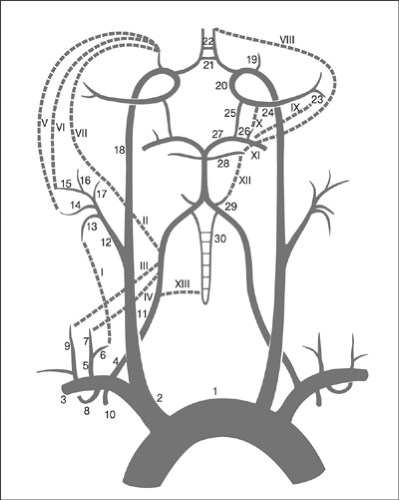
The target common carotid artery must be assessed to determine the angle of the take-off, level of bifurcation, and presence of associated lesions. The external carotid artery is assessed mainly in terms of its branching pattern in order to identify a suitable branch for guidewire placement, evidence of abnormal vessel connections, embryonic bridges, or collateralization (note of warning: risk of distal and intracranial embolization), and evidence of previous embolic events. The internal carotid artery should be studied along its entire course to determine its course, branching pattern, the presence of main branches, collateral vessels (including its participation in the formation of the circle of Willis), and the presence of abnormal flow patterns (including competitive flow), and to identify associated anomalies such as embryonal vessels, the presence of associated lesions, the anatomic position of the take-off of the ophthalmic artery, and the form of the carotid T in order to estimate the risks of traumatization that are mainly due to guidewire or distal protection device (DPD) manipulation. Tortuosities, coilings, and kinkings may be associated with stenosis, and several projections could be required to define the anatomy. The proximity of the convoluted segments to the target lesion may affect the operator’s ability to safely deploy the DPD. In addition, excessive meandering and elongation of the internal carotid artery may be increased if a stent is placed with suboptimal longitudinal flexibility. To estimate the effect of stenting, imaging of the internal carotid artery in several different positions of the head may be helpful. Figure 7-2 shows the common collateral pathways of the cerebrovascular circulation as a potential source of intracranial embolization.36
The target lesion and the adjacent distal and proximal segments of the internal carotid artery are assessed for stent sizing, to measure the severity of the stenosis, and to define the morphology of the target lesion. The severity of the internal carotid artery stenosis is measured as a percentage reduction in the diameter of the patent lumen at the narrowest segment. Most frequently, automatic edge detection algorithms or densitometric quantitative techniques are employed; alternatively the measurements can be performed manually using calipers. Because of significant changes in size of the internal carotid artery at the bulbar level, precise definition of the employed
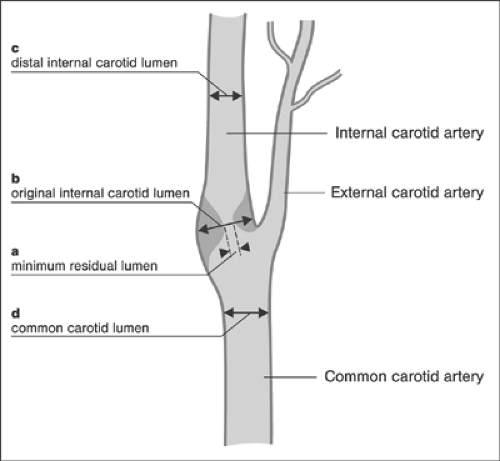
methodology is required to allow reproducible measurements. According to the NASCET method, the shortest distance between the two opposing leading edges at the narrowest point and the lumen diameter of the adjacent healthy segment distal to the stenosis are compared. According to the European Carotid Surgery Trial (ECST) method, the luminal and the assumed nominal diameters at the site of maximum stenosis are compared. Finally, using the common carotid (CC) method, the minimum luminal diameter is compared with the luminal diameter in the proximal common carotid artery. Although the use of each of these three different techniques leads to the calculation of different absolute numbers for stenosis severity, the results are consistent, maintaining an almost linear relationship, and the equivalence of measurements has been determined: 50% NASCET stenosis is equivalent to a 65% ECST and CC stenosis, and 70% NASCET stenosis is equivalent to a 82% ECST and CC stenosis (for review, see references 24 and 37). Figure 7-3 shows a schematic comparison of quantitative methods to assess the severity of internal carotid artery stenoses. The principal morphologic aspects of the target lesion that are relevant to CAS include the presence of thrombi, surface morphology (ulcerations), calcifications, and the plaque burden.
methodology is required to allow reproducible measurements. According to the NASCET method, the shortest distance between the two opposing leading edges at the narrowest point and the lumen diameter of the adjacent healthy segment distal to the stenosis are compared. According to the European Carotid Surgery Trial (ECST) method, the luminal and the assumed nominal diameters at the site of maximum stenosis are compared. Finally, using the common carotid (CC) method, the minimum luminal diameter is compared with the luminal diameter in the proximal common carotid artery. Although the use of each of these three different techniques leads to the calculation of different absolute numbers for stenosis severity, the results are consistent, maintaining an almost linear relationship, and the equivalence of measurements has been determined: 50% NASCET stenosis is equivalent to a 65% ECST and CC stenosis, and 70% NASCET stenosis is equivalent to a 82% ECST and CC stenosis (for review, see references 24 and 37). Figure 7-3 shows a schematic comparison of quantitative methods to assess the severity of internal carotid artery stenoses. The principal morphologic aspects of the target lesion that are relevant to CAS include the presence of thrombi, surface morphology (ulcerations), calcifications, and the plaque burden.
On the basis of the anatomic information derived, the technical feasibility and strategy of CAS are formulated and potential interventional risks defined. The main issues include the estimated stability and backup of the support system required to overcome the anatomic adversities of the proximal vascular pathways, definition of the relevant external carotid artery branch for safe engagement of the support system, and evaluation of the interventional site, including the configuration, topography, and geometry of the flow divider (stent sizing and positioning), and features of the target lesion critical to guidewire use (position and diameter of the residual lumen with respect to the walls of the carotid bulb, and presence of ulcerations and tissue pockets), crossing of the DPD, and the need for predilatation (severity, calcification). Safe crossing ability of the DPD and adequate distal spacing between the target lesion and DPD are critical to overall procedural risk assessment.
To ensure quality, close adherence to established standards regarding procedural outcomes is required.32 Although the achievement of perfect outcomes, that is, 100% success with 0% complications, is desirable, a certain level of complications may be considered acceptable on the basis of good clinical practice standards. The definition of thresholds of procedural indicators of outcome serves benchmarking. To allow consistent reporting of outcomes, unequivocal definition of procedural indicators such as complications is mandatory (Table 7-3). The suggested thresholds of neurologic and nonneurologic complications in performing quality, adult, diagnostic neuroangiography, as proposed by joint effort of the Society of Interventional Radiology, the American Society of Interventional and Therapeutic Neuroradiology, and the American Society of Neuroradiology, are summarized in Tables 7-4 and 7-5. To define the degree of severity of neurologic complications, modified Rankin disability score should be determined and reported (Table 7-6).
Diagnostic cerebrovascular angiograms should be evaluated by an interventionist, a vascular surgeon, and a neurologist; to select the optimum strategy of treatment, i.e., medical versus carotid artery endarterectomy (CAE) versus CAS, a consensus decision of the whole team is required. Provided that there exists a comparable level of interventional and surgical competence at a given institution, the indications for specific means of revascularization (CAE vs. CAS) depend primarily on estimates of the interventional and surgical risk (e.g., comorbidities, need
for coronary artery bypass graft [CABG] surgery), presence of hostile neck conditions, and the angiographic substrate of the target lesion.
for coronary artery bypass graft [CABG] surgery), presence of hostile neck conditions, and the angiographic substrate of the target lesion.
TABLE 7-3. Definition of Outcomes: Neurologic Complications, Minor and Major Complications | ||
|---|---|---|
|
TABLE 7-4. Procedural Outcome Threshold of Neuroangiography in Adults: Neurologic Complications | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
TABLE 7-5. Procedural Outcome Threshold of Neuroangiography in Adults: Nonneurologic Complications | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
TABLE 7-6. Modified Rankin Disability Score | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Revascularization Options
It is customary in clinical practice to differentiate between symptomatic and asymptomatic carotid artery stenosis. The definition is based on the patient’s history and physical examination; patients with documented carotid artery stenosis but no history of focal neurological signs and symptoms are considered asymptomatic, including those with detected “silent” infarcts on CT or MR imaging, regardless of the size and number of ischemic defects! In clinical practice, however, patients with radiographic evidence of ischemic defects in the distribution of a stenotic internal carotid artery are considered to have an active disease associated with a greater need for secondary prevention, including revascularization. The principal revascularization options are carotid artery surgery or CAS.
In 1954, Eastcott and colleagues published the first case report on revascularization of the carotid artery by resecting the 3-cm-long stenotic segment and repairing it using end-to-end anastomosis.38 The first CAE was performed in 1956, as reported by DeBakey.39 Evolution of carotid artery surgery for stroke prevention has been extensively reviewed and reported in the literature.6,40
It was not until 1991 that the benefits of CAE in symptomatic patients were reported in two large prospective and randomized trials, namely, ECST41 and NASCET,42 with final results and recommendations of both trials reported in 1998.43,44 Recently, the evidence of data supporting the use of CAE for symptomatic carotid disease in relation to subgroups has been reviewed and presented.45 Evidence of benefits of CAE in patients with asymptomatic carotid disease has been derived primarily from two large trials, namely, the Asymptomatic Carotid Atherosclerosis Study (ACAS)46 and the Asymptomatic Carotid Surgery Trial (ACST)47; both trials have recently been commented on and reviewed.48
It was not until 1991 that the benefits of CAE in symptomatic patients were reported in two large prospective and randomized trials, namely, ECST41 and NASCET,42 with final results and recommendations of both trials reported in 1998.43,44 Recently, the evidence of data supporting the use of CAE for symptomatic carotid disease in relation to subgroups has been reviewed and presented.45 Evidence of benefits of CAE in patients with asymptomatic carotid disease has been derived primarily from two large trials, namely, the Asymptomatic Carotid Atherosclerosis Study (ACAS)46 and the Asymptomatic Carotid Surgery Trial (ACST)47; both trials have recently been commented on and reviewed.48
On the basis of current American Heart Association (AHA) guidelines, CAE appears beneficial for symptomatic patients with ipsilateral, 70% to 99% carotid artery stenosis, with a reported complication rate of ≤3%, whereas the indication for a 30% to 69% stenosis (measured by NASCET criteria) remained uncertain; it also appears beneficial for asymptomatic patients with carotid stenosis of ≥60%, a surgical risk of ≤3%, and a life expectancy of ≥5 years, irrespective of the status of the contralateral carotid artery (proven indication), as well as in patients with carotid stenosis of ≥60%, irrespective of the status of the contralateral carotid artery, who are scheduled for simultaneous CABG surgery (acceptable indication).49 However, the latter strategy, which was introduced in 1972,50 has remained a point of ongoing controversy.51 On the basis of the recommendations of the American Academy of Neurology, the indications for CAE for severe (70% to 99%) symptomatic stenosis were endorsed. Moderate benefits were assigned to treatments of patients with a 50% to 69% symptomatic stenoses, and no indications were seen for patients with <50% stenosis, provided in all cases that the perioperative combined stroke and death rate was <3%. In patients with asymptomatic 60% to 99% stenosis the Academy saw fewer benefits than for symptomatic patients, and individual decisions were recommended.52 CAE is presently the standard treatment for patients with symptomatic and asymptomatic extracranial carotid artery stenosis.
Percutaneous intervention for carotid artery stenosis in humans was first reported by Mathias in 1977.53 Subsequently, sporadic studies on carotid balloon angioplasty were published, reporting technical success rates between 79% and 98%, and a risk of stroke between 4% and 6%.54,55,56,57 In 1994, the first stent-supported carotid angioplasties using Palmaz medium biliary stents (Cordis, Miami, FL, USA), Flex-Stents (Cook, Bloomington, IN, USA), and Wallstents (Schneider, Zurich, Switzerland) were performed and reported.58 Introduction of distal protection devices (DPDs) in 1996 59 has increased procedural safety and has reduced the incidence of neurological complications.60,61,62 Subsequently, in a number of single- and multicenter studies, improved endovascular techniques and technology have been used and CAS efficacy documented.63 On the basis of early results and evolving evidence, an early advisory AHA statement64 and more recent extensive guidelines for CAS performance have been formulated.65
Considering the indications for CAE in symptomatic and asymptomatic patients (Table 7-7) and on the basis of the evolving evidence available in 2003, the Collaborative Panel of the American Society of Interventional and Therapeutic Neuroradiology, the American Society of Neuroradiology, and the Society of the Interventional Radiology proposed indications and contraindications for CAS (Table 7-8).65
TABLE 7-7. Suggested Indications for Carotid Endarterectomy | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Carotid Artery Stenting: a Coronarylike Approach
Within less than a decade, important advances have been made in dedicated CAS instrumentation, including the introduction of high-performance 0.035-in. and 0.014-in. guidewires, low-profile dilatation balloons, stents with improved design that provide greater radial force, better crossing, and scaffolding properties, and more efficacious and less traumatic distal protection devices. In addition, the coronarylike approach to CAS has been developed, which consists of telescopic coaxial techniques using low profile instrumentation to provide direct access to the target lesion and coronary equipment and rapid exchange techniques to accomplish the revascularization.66,67
With CAE representing the standard treatment of carotid artery disease, the rationale for CAS is to provide treatment options for patients with high surgical risk, patients with a “hostile neck” (i.e., high, above C2, and low, below the clavicle, internal carotid artery stenosis, prior neck radiation or neck surgery, medical conditions associated with cervical spinal immobility, extreme obesity with short neck, and requirements for tracheostomy), and patients with conditions associated with poor surgical outcomes (e.g., restenosis following an ipsilateral CAE). In addition patients with high surgical risk are considered. High surgical risk has been variously defined in the literature; those groups considered at such risk included patients with coronary artery disease requiring CABG surgery; patients with ongoing stable or unstable angina despite medication; patients with recent myocardial infarction, that is, within the past 30 days; patients with congestive heart failure, uncontrolled hypertension, contralateral carotid occlusion, renal insufficiency, or creatinine >1.5 mg/dL; and those with poorly controlled diabetes.68,69,70,71
To date, only one prospectively randomized trial study design has allowed direct comparison of the state-of-the-art coronary-approach CAS with CAE.72,73 In this trial, 747 patients at 29 sites were evaluated between 1999 and 2002 for revascularization of internal carotid artery stenosis. A total of 334 patients were randomized, and 310 patients were treated; 406 patients were treated in the nonrandomized CAS, and seven patients in the nonrandomized CAE arm. Inclusion and exclusion criteria are
summarized in Tables 7-9 and 7-10. The primary end points were death (all causes), any stroke, and myocardial infarction ≤30 days postprocedure, and death (all causes) and ipsilateral stroke between days 31 and 360 postprocedure.
summarized in Tables 7-9 and 7-10. The primary end points were death (all causes), any stroke, and myocardial infarction ≤30 days postprocedure, and death (all causes) and ipsilateral stroke between days 31 and 360 postprocedure.
TABLE 7-8. Suggested Indications and Contraindications for Carotid Artery Stenting (CAS) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
TABLE 7-9. SAPPHIRE Trial: Inclusion Criteria | |||||
|---|---|---|---|---|---|
|
The technical success for the stent delivery system (Precise, nitinol stent, Cordis Corp., Miami Lakes, FL, USA) was 99.4% (<50% residual stenosis) and 91.2% (<30% residual stenosis). The technical success rate for the ultimate placement rate of the protection system (Angioguard-XP, Cordis Corp., Miami Lakes, FL, USA) was 98.1%.
For the CAS and CAE groups, the cumulative incidence of death at 1 year was 7.4% versus 13.5%, and the cumulative incidence of stroke was 6.2% versus 7.9% at 1 year, respectively. The combined incidence of major adverse events (MAEs) between the two groups was 4.8% versus 9.8%, respectively. The incidence of MAE at 30 days and at 360 days is shown in Figures 7-4 and 7-5,
respectively. Figure 7-6



respectively. Figure 7-6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree