Class
Examples
Cancers treated
Cardiotoxicity
Anthracyclines
Doxorubicin, epirubicin
Acute leukemia, Hodgkin’s and non-Hodgkin’s lymphoma, breast cancer
Acute: HF, arrhythmias, QT changes
Chronic (dose-dependent): HF nonreversible
Alkylating agents
Cyclophosphamide
Blood, breast, endometrial, bladder cancer
Reversible HF, pericardial effusion, arrhythmias
Antimicrotubule agent
Paclitaxel, docetaxel
Breast and ovarian cancer, Kaposi’s sarcoma, prostate and bladder cancer, gastric adenocarcinoma
Bradycardia, myocardial ischemia, syncope, LV dysfunction, ventricular arrhythmias
Antimetabolites
Cisplatin, 5-fluorouracil
Solid tumors (lung, colon, breast)
Myocardial ischemia, myocardial infarction, arrhythmias
Vinca alkaloids
Vincristine, vinblastine
Leukemia, lymphoma
Myocardial ischemia
Tyrosine kinase-inhibiting antibodies
Trastuzumab, bevacizumab, rituximab, alemtuzumab
Breast and lung cancer, metastatic colorectal cancer, lymphomas, leukemias, transplant rejection
Reversible LV dysfunction, venous thrombosis, systemic hypertension
Small-molecule tyrosine kinase inhibitors
Imatinib, sorafenib, lapatinib, sunitinib
Renal cell cancer, lung and pancreatic cancer, leukemia
LV dysfunction, myocardial infarction, pericardial effusion, HF
Others
Tamoxifen, arsenic trioxide, retinoic acid, thalidomide
Breast cancer, multiple myeloma, melanoma, metastatic renal cell carcinoma
Deep-vein thrombosis, pulmonary embolism, stroke, myocarditis
In addition to traditional cardiotoxic agents, such as anthracyclines or radiation-related heart disease, newer therapies including tyrosine kinase inhibitors [4–6] and even therapies that are not necessarily classified as “chemotherapy” may also promote CV disease or events. For example, the administration of hormone deprivation therapies, which have dramatically reduced cancer recurrence and improved survival in women with breast cancer and men with prostate cancer, is now increasingly associated with CV events [7, 8].
Obviously, a chemotherapeutic agent can induce CV toxicity through multiple mechanisms and change their effect in relation to the combination of the antiblastic therapy.
In Table 19.1 the CV effect of a single agent is described, and we analyze the most common clinical syndrome related to its CV toxicity (Table 19.2).
Table 19.2
Common clinical syndromes related to chemotherapeutic agents
Drugs associated with CHF | Anthracycline Cyclophosphamide Tyrosine kinase-inhibiting antibodies |
Drugs associated with ischemia or thromboembolism | Antimetabolites Antimicrotubule agent Cisplatin Thalidomide |
Drugs associated with hypertension | Bevacizumab Cisplatin Sorafenib |
Hemorrhagic myocarditis (rare) | Cyclophosphamide |
Bradyarrhythmias | Paclitaxel |
Raynaud’s phenomenon | Vinblastine |
QT prolongation | Arsenic trioxide |
Pulmonary fibrosis | Methotrexate |
19.1 Congestive Heart Failure
For Left ventricular dysfunction means a global decrease in LVEF or more severe in the septum; symptoms of congestive heart failure (CHF) include decline of the left ventricular ejection fraction (LVEF) at least 5% to less than 55% with signs or symptoms of CHF, or at least 10 to 55% without signs or symptoms [9, 10]. The most common form of cardiotoxicity is anthracycline-related CHF.
19.1.1 Anthracycline Cardiotoxicity
Doxorubicin cardiotoxicity varies from 4 to 36% depending on the dose; epirubicin and idarubicin have a lower incidence of CHF.
Free radical formation is generally accepted as the main mechanism; apoptosis also plays a prominent role in the myocardial cell loss that has been demonstrated in such cases. Risk factors for AC are high, single intravenous dose; time of drug infusion <30 min; history of previous irradiation; use of other concomitant agents such as cyclophosphamide, trastuzumab, and paclitaxel; female gender; and young or old age.
There is an acute effect with a transient decline in myocardial contractility after infusion and a chronic progressive effect presenting as dilated cardiomyopathy is typically manifested as clinical heart failure or subclinical decline in myocardial function, and may present early, within 1 year of the termination of chemotherapy, or late-delayed, becoming evident beyond 1 year post-treatment. The chronic cardiomyopathy is related to myocardial necrosis and vacuolization, caused by the suppression of the synthesis of DNA, RNA, proteins, and transcription factors. Reduced protein expression results in disruption of sarcomeric proteins and myofilaments. Anthracycline also alters the adenylyl cyclase activity and calcium homeostasis disrupting the dynamic regulation of cardiac function [11–13].
19.1.2 Trastuzumab (Biological Therapies)
This is a monoclonal antibody that binds to the extracellular domain of HER2 (human epidermal growth factor receptor 2), inhibiting signal transduction. The HER2 gene is overexpressed in 15–20% of breast cancer cases, with overproduction of HER2.
The treatment with this type of therapy results in higher rates of cardiac dysfunction if combined with a high dose of anthracyclines (>300 mg/m2).
The cardiotoxicity is related to the role of HER2 in cardiomyocyte survival and development [14, 15]: the cellular level overexpression of HER2 and/or neuregulin (NRG)-mediated activation of the HER2/HER4 signaling pathway protect against oxidative stress and prevent apoptosis. High serum levels of HER2 have been detected in individuals with chronic HF [16, 17] and clinical trials have shown that administration of recombinant human NRG-1 improves cardiac function in chronic HF and is well tolerated [18]. Thus, although cardiac stress leads to increased HER2 expression and HER2/HER4 activation by NRG, for example, during anthracycline therapy with pressure overload, inhibition of HER2 by trastuzumab induces ventricular dysfunction, developing dilated cardiomyopathy. The concomitant use of trastuzumab and anthracyclines increases ROS levels, and creates an oxidative stress that causes overexpression of angiotensin II, which inhibits the action of NRG and its binding to HER, blocking anti-apoptotic pathways. Moreover, the Ang II, through activation of NADPH oxidase, produces superoxide anion radicals (potent ROS; Fig. 19.1).
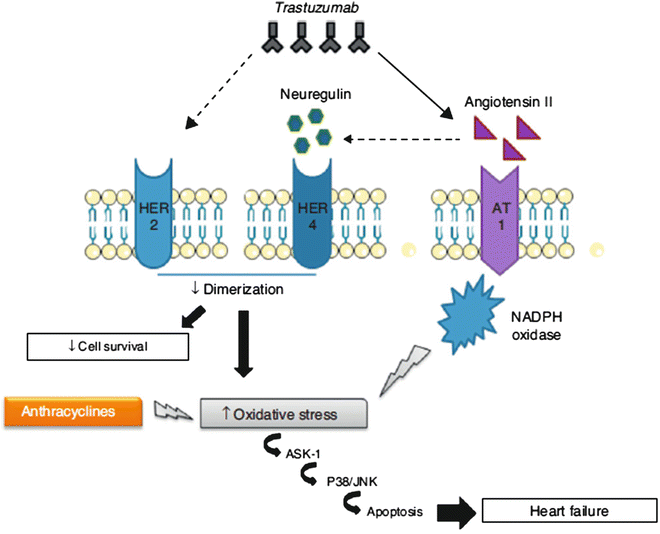

Fig. 19.1
Mechanism of action of trastuzumab
19.1.3 Tyrosine Kinase Inhibitors (Biological Therapies)
Tyrosine kinase inhibitors (TKIs) are small-molecule targeted therapeutics that are directed against specific molecules and signaling pathways [21]. The drugs in this class are similar; they differ in their specific targets or combination of targets and thus result in a variety of toxicities.
For example, CHF caused by sunitinib may be related to mitochondrial damage in cardiomyocytes or activation of apoptosis and interference in cellular metabolism. CHF related to the use of lapatinib may be a result of HER2 inhibition [22].
19.2 Systemic Hypertension
The principal mechanism involved is the inhibition of vascular endothelial growth factor (VEGF) by anti-angiogenic cancer drugs (i.e., bevacizumab, sunitinib, and sorafenib), which lead to endothelial cell dysfunction and defects within the vascular lining, resulting in activation of tissue factor, thus leading to an increased risk for thromboembolism. In addition, inhibition of VEGF may cause a reduction in nitric oxide and prostacyclin, which promotes vasoconstriction and increases peripheral vascular resistance [21, 22].
19.3 Myocardial Ischemia: Fluoropyrimidines
The pathogenesis of cardiotoxicity is unknown; the mechanisms involved are probably coronary vasospasm, arterial thromboembolic events, arteritis, interaction with the coagulation system, and direct toxicity on myocardium, such as the accumulation of metabolites that interfere with cellular metabolism and apoptosis leading to inflammatory lesions that could mimic myocarditis. Clinical presentations are stable angina, and myocardial infarction more often in patients with pre-existing coronary atherosclerosis. The drugs particularly involved are fluoropyrimidines (such as 5-fluorouracil), bevacizumab, anti-microtubule agents (paclitaxel and docetaxel), tyrosine kinase inhibitors (sorafenib and sunitinib), and the Vinca alkaloids vincristine and vinorelbine [6, 26]
Toxicity appears to be dose-dependent and infusion-rate-dependent. The percentage of clinical presentation for angina is 45%, for myocardial infarction 22%, for arrhythmias 23%, for acute pulmonary edema 5%, for cardiac arrest 1.4%, and for pericarditis 2%, with a mortality rate of 2.2–13% in the literature [27].
19.4 Arrhythmias
The chemotherapeutic agents known to cause arrhythmias are anthracyclines (doxorubicin and epirubicin), anti-microtubule agents (paclitaxel and docetaxel), antimetabolites (capecitabine, 5-flurouracil, and gemcitabine), alkylating agents (cisplatin and cyclophosphamide), tyrosine kinase inhibitors (trastuzumab and cetuximab), arsenic trioxide, thalidomide, and interleukin 2.
The most frequent arrhythmia registered is atrial fibrillation with to unknown and variable incidence related to treatments. Cancer itself causes arrhythmias independently of pre-existing risk factors that obviously increase morbidity (advanced age, radiotherapy of the heart, the presence of amyloid infiltration, and any underlying conduction system disturbance) [28].
19.5 Thromboembolism
Venous thromboembolism is a leading cause of death in cancer patients and is associated with the use of anti-angiogenic drugs, thalidomide, lenalidomide, bevacizumab, and hormone therapy such as tamoxifen. The thrombogenic mechanism of these drugs involves direct action on endothelial cells and increased platelet aggregation.
19.6 Radiotherapy
Another type of anticancer therapy is radiation, which is administered in about 50% of patients with cancer. The groups of patients in which radiation-associated cardiac injuries are of clinical importance are those with curable malignancies (mainly Hodgkin’s lymphoma and early-stage breast cancer, lung, and esophageal cancer) irradiated at a relatively younger age; thus, there is enough time to develop clinically significant late cardiac injury. The extent of cardiotoxicity depends mainly on radiation dose, the area of the heart exposed, and the particular technique applied. Risk factors for radiation-associated heart damage include dose >30–35 Gy, a large volume of irradiated heart, younger age at exposure, longer time since exposure, use of cytotoxic chemotherapy, endocrine therapy or trastuzumab, and the presence of other risk factors such as diabetes, hypertension, dyslipidemia, obesity, and smoking [29].
The mechanism of cardiotoxicity is likely secondary to the generation of reactive oxygen species, which disrupt DNA strands and lead to vascular endothelial damage, and inflammation, which leads to fibrosis [30, 31].
RT heart injury can cause various cardiac syndromes such as the following:
Arteritis of the endothelium of coronary arteries, which can cause premature coronary artery disease and atherosclerosis mainly in the left anterior descending and right coronary artery, 10–15 years after RT.
Acute pericarditis and symptomatic (hemodynamic compromise with constriction or tamponade) or asymptomatic chronic pericardial effusion usually appear 6–12 months following RT.
Myocarditis and congestive heart failure due to nonspecific diffuse interstitial fibrosis.
Valvular stenosis and regurgitation mainly of mitral and aortic valves.
Fibrosis of the conduction system and disturbed heart rate and complete or incomplete heart block.
19.7 Monitoring and Non-Invasive Diagnosis
Patients undergoing anticancer therapy should follow standard guidelines for reducing CV risk, such as blood pressure control, lipid-level reduction, smoking cessation, and lifestyle modifications.
Baseline clinical evaluation, ECG evaluation, and periodic monitoring of cardiac function with echocardiography are considered according to the type of patients and chemotherapeutic treatment.
There are recent research initiatives suggesting the possible utility of non-invasive imaging technologies for identifying subclinical CV injury in those receiving treatment for and surviving cancer.
19.7.1 Role of Echocardiography
In addition to evaluating LV structure, echocardiography provides information on both systolic function and diastolic function; also, recent techniques have become available to measure myocardial deformation, including LV strain, strain rate, or twist and torsion that may provide a new understanding regarding the early stages of the pathophysiology of cardiac dysfunction upon receipt of cancer treatment [32, 33]. Moreover, echocardiography provides additional information about valvular function and pericardial fluid/physiology that might occur after cancer treatment.
The m-mode, Doppler, and 2D and 3D information is useful, not only for diagnosing a cardiac injury, but also often to predict the cardiotoxicity. For example, some studies have shown that LV diastolic properties, such as a decrease in the E/A ratio, or prolongation of IVRT, or the deceleration time of early diastolic filling, can predict doxorubicin-induced LV systolic dysfunction [34]. Some authors demonstrated that a reduction of more than 10% of global and regional longitudinal and radial strain in the first weeks of treatment with anthracycline and trastuzumab predicts the later development of a reduction of LVEF 6 months after the initiation of these therapies [32, 35] with a sensitivity of 78% and specificity of 79%, and a negative predictive value of 93%.
Other studies try to determine the prognostic utility of other echocardiographic measures, such as the twist and torsion of the LV in those treated for cancer, and this may be the expression of myo-filament disorganization and cardiomyocyte necrosis.
19.7.2 Role of Cardiovascular Magnetic Resonance Imaging
Cardiovascular magnetic resonance (CMR) imaging gives information about cardiac and vascular anatomy, tissue characteristics (presence of fibrosis, inflammation, injury, etc.), left and right ventricular systolic or diastolic function, blood flow, and myocardial perfusion or metabolism for the purposes of understanding the etiology of LV systolic or diastolic dysfunction. For this reason, the American College of Cardiology/American Heart Association recognize CMR imaging as a method of identifying CV dysfunction after treatment for cancer and have incorporated it across research studies to define the pathophysiology of cancer treatment-related CV toxicity. By CMR imaging the presence and severity of morphological and functional abnormalities of the LV or RV myocardium can be identified and understood, determining the underlying etiology (e.g., ischemic versus non-ischemic disease) of LV or RV dysfunction, and identifying prognostic factors related to patient outcomes. CMR offers more accurate assessment of function and morphology than most available imaging modalities, providing reliable volumetric data with high diagnostic image quality in nearly all patients. Table 19.3 displays quantitative and qualitative parameters, each of which can be used as diagnostic markers or descriptors in patients with suspected heart failure [36].
Table 19.3
Cardiovascular magnetic resonance imaging-derived parameters in patients with suspected heart failure
Parameters | |
|---|---|
Systolic function | LV and RV end-diastolic volume and indices |
LV and RV end-systolic volume and indices | |
LV and RV stroke volume and ejection fraction | |
Cardiac output and cardiac index | |
Regional and global measures of myocardial strain | |
Morphology | LV mass and indices |
Mean and maximum myocardial wall thickness | |
Assessment of pericardium | |
Wall stress | End-systolic wall stress |
Diastolic function | Circumferential strain and strain rate |
End-diastolic forward flow in pulmonary veins | |
E/A ratio | |
Reversible acute injury | Edema |
Irreversible injury | Myocardial fibrosis |
19.7.3 Role of Cardiovascular Computed Tomography
Cardiovascular CT may be useful for evaluating the pericardium of patients who received radiation or surgical treatments; to identify abnormal thickening and calcification of the pericardium; and to measure coronary artery calcium or directly visualize the coronary arteries. There are currently insufficient data to recommend the routine use of coronary CT angiography or calcium scoring in patients who underwent high-dose radiation therapy. In addition, the presence of coronary artery calcification before treatment for cancer has not been shown to predict future CV risk upon receipt of chemotherapy, tyrosine kinase inhibitors, or radiation therapy.
19.7.4 Role of Nuclear Medicine Imaging
Today, equilibrium radionuclide angiography (ERNA) is used to measure LV function through determination of the LV ejection fraction (LVEF). LV diastolic function is often assessed using radioisotope-based techniques. Count–time curves, the peak filling rate (PFR), the PFR normalized to stroke volume, and time-to-peak filling rate are detected with planar equilibrium radionuclide ventriculography (ERNV). For example, a reduction in these ERNV measures of LV diastolic function correlates with the simultaneous decreases in LVEF, suggesting an impairment of systolic and diastolic function during anthracycline therapy [37, 38]
19.7.5 Role of Biomarkers
Early identification of patients at risk for cardiotoxicity represents a primary goal for cardiologists and oncologists; we know the usefulness of LVEF monitoring in patients who have undergone chemotherapy, but it is not specific and sensitive enough to predict damage, because it permits the identification of cardiac damage only after the onset of cardiac dysfunction. An alternative diagnostic strategy is the use of troponin and cardiac peptides in the early detection of cardiotoxicity in clinical practice. Measurement of EF may underestimate actual cardiac damage, because patients may experience subtle changes in cardiac function not detected on imaging studies. Serum cardiac biomarkers, such as N-terminal prohormone brain natriuretic peptide and/or troponin, may also play a role in the detection of cardiac damage, but further investigation is needed to classify their predictive value.
The mechanisms responsible for troponin release after chemotherapy are still being defined. Results of many studies support the non-ischemic etiology of the serum troponin increase after chemotherapy [39]. Furthermore, the persistence of cTnI elevation, observed in some studies, 1 month or more after the end of chemotherapy, suggests the occurrence of a release pattern different from ischemic injury [40]. In acute coronary syndromes, indeed, troponin typically returns to baseline within 10 days and is associated with, not followed by, ventricular dysfunction [41].
Troponin determination detects the presence of cardiotoxicity very early, before impairment of cardiac function, and can be revealed by any other diagnostic technique. Immediately after the last dose of chemotherapy, determination of troponin allows for the discrimination of patients at a high risk for cardiotoxicity from patients at low risk; among patients with positive troponin values, persistence of the increase 1 month after the last administration of chemotherapy is related to an 85% probability of major cardiac events within the first year of follow-up [39, 42, 43], whereas a persistently negative troponin test result can identify, with a predictive negative value of 99%, patients with the lowest risk for cardiotoxicity (Fig. 19.2).
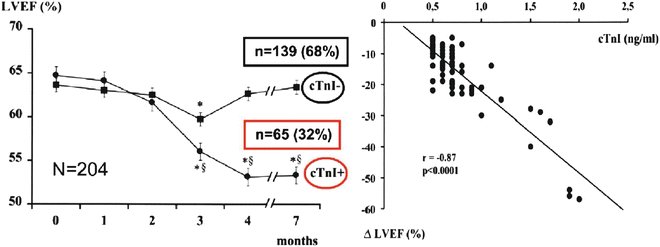

Fig. 19.2
Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. Cardinale et al. [42]
Although the role of Tn is clear, for the cardiac natriuretic peptides (CNPs), such as B-type natriuretic peptide (BNP) and the amino-terminal fragment of its precursor (NT-proBNP), it is not the same thing. They represent efficient markers of ventricular dysfunction as they are rapidly produced and secreted by the heart in response to ventricular wall distention. There are no definitive results regarding the role of CNPs in assessing cardiotoxicity in clinical practice.
19.8 Treatment and Prevention
Drugs used to treat heart failure have also shown promise in the prevention of chemotherapy-induced cardiotoxicity; early detection of cardiotoxicity is of crucial importance ineffectively preventing or treating patients in a phase in which the disease is still reversible. Jensen, in some studies, considered around 108 patients with anthracycline cardiomyopathy only, who had symptoms of heart failure. Forty-six patients (43%) were treated with digitalis and diuretics, and 32 patients (30%) were treated with different angiotensin-converting enzyme inhibitor (ACEIs; enalapril in most cases); among them, only 13 patients received ACEIs as a first treatment. Finally, only 5 patients (5%) were treated with beta-blockers alone (carvedilol in most cases), and only 25 patients (23%) received a combination of both these classes of drugs. Therefore, no clear evidence can be obtained from these findings in terms of defining the best therapeutic strategy for this CMP [44– 47].
A study of 50 patients randomly assigned to receive prophylactic carvedilol or placebo before anthracycline chemotherapy found that beta-blockers preserve EF [48]. Similar results were seen with nebivolol [49]. A larger randomized trial that included 473 patients demonstrated that enalapril may also help prevent cardiotoxicity; however, the mean age was 45 years, and the benefits in the elderly are not known [43].
Cardinale et al. [50] demonstrated that early treatment is particularly critical in asymptomatic patients. Indeed, most responders were either asymptomatic or had a low New York Heart Association (NYHA) functional class at the time of HF therapy initiation (Fig. 19.3).
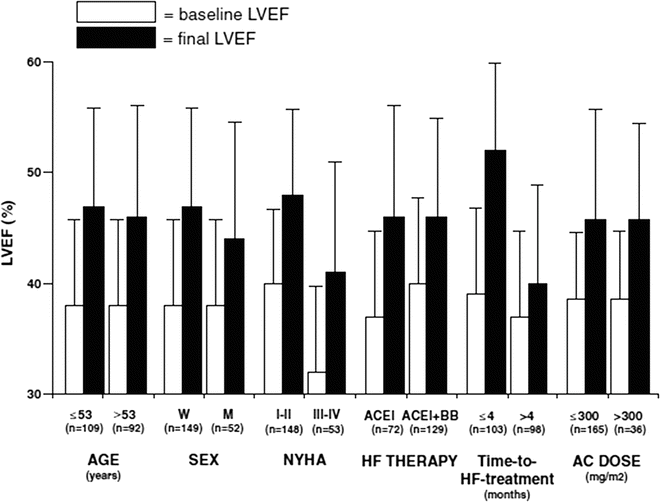

Fig. 19.3




Left ventricular ejection fraction (LVEF) before (baseline) and after (final) heart failure (HF) therapy in various subgroups of patients. For age, time-to-HF treatment, and anthracycline (AC) dose, patients were stratified according to the median value. p<0.001 for all comparisons. ACEI angiotensin-converting enzyme inhibitor, BB beta-blocker, M men, NYHA New York Heart Association, W women
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


