Fig. 8.1
HeartMate III fully magnetically levitated left ventricular assist system
The rotor is fully supported by magnetic levitation, obviating mechanical or fluid bearings. Both drive (i.e., rotation) and levitation of the rotor are accomplished using a single stator comprising iron poles, copper coils, and position sensors. By measuring the position of a permanent magnet in the rotor and appropriately controlling the current in the drive and levitation coils, the radial position and rotational speed of the rotor are actively and independently controlled. Because of the permanent magnet’s attraction to the iron pole pieces, the rotor passively resists excursion in the axial direction, whether translating or tilting.
This magnetic levitation technology essentially eliminates rotor mechanical wear as a reliability factor and facilitates relatively large gaps between the turning rotor and the stationary housing (Fig. 8.2). These gaps are approximately 0.5 mm radially and 1.0 mm axially, 10–20 times greater than those in a hydrodynamic bearing. Computational fluid dynamic (CFD) analysis confirms that flow fields are well organized across wide ranges of flow (2–10 L/min) and that surface shear forces are kept lower than other types of pumps (Fig. 8.3). An additional benefit of magnetic levitation is that these large gaps are maintained irrespective of rotor speed, even when not turning. Thus, it is conceivable to operate at lower speeds, which might be important in future applications such as partial left ventricular assistance, right ventricular assistance, or weaning exercises.
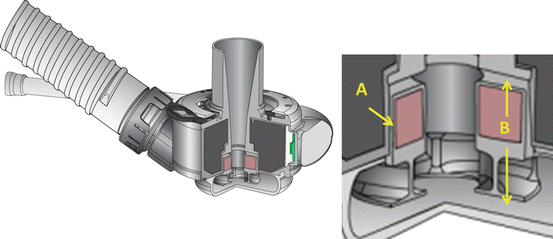
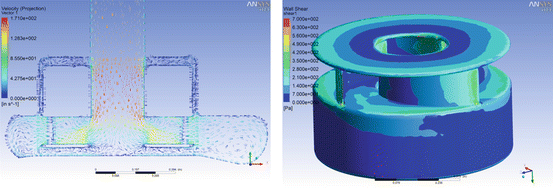

Fig. 8.2
Cross section of HeartMate III LVAD . Wide gaps for secondary flow paths of 0.5 mm radially (a) to 1.0 mm axially (b) are possible with full magnetic levitation design

Fig. 8.3
Computational fluid dynamics (CFD) analysis of HeartMate III blood pump illustrates low shear, well-organized flow fields, smooth flow transitions, and the avoidance of regions of stasis over wide ranges of flow
In blood, large gaps are advantageous in several ways. Low hydraulic resistance ensures avoidance of stasis in those regions. Low shear stresses reduce trauma to erythrocytes and circulating proteins, which could beneficially affect adverse events such as thromboembolism, hemolysis, and bleeding. Further, because minor radial and axial deviation of the rotor is tolerable, levitation is readily maintained through the most rigorous patient activities, and a novel feature called an artificial pulse is facilitated. As for other rotary pumps, with HeartMate III the clinician will set only a single speed, w c in Fig. 8.4; however, the rotor speed will periodically depart from this value in order to contribute a flow disruption that in some ways mimics native cardiac contractility. This artificial pulse “beats” 30 times per minute, asynchronously with the heart.
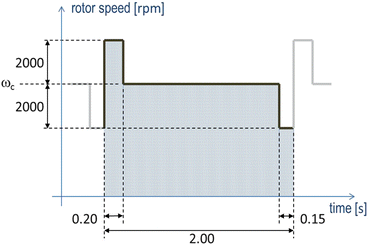

Fig. 8.4
Schematic of the changes in rotor speed above and below the set speed w c to create the HeartMate III artificial pulse
Although it has been well established with continuous-flow LVADs that an MCS device does not need to pulse for survival, there are theoretical advantages in contributing or enhancing the native pulsatility in various conditions [6]. Indeed, there are distinct benefits in producing unsteadiness in the flow within any rotary pump, and there may be similar benefits external to the pump, reducing adverse events such as aortic insufficiency and bleeding, for example.
To further optimize hemocompatibility , in addition to reducing shear and increasing washing, all the blood-contacting titanium surfaces except for the rotor and rotor well are coated with sintered titanium microspheres. Surfaces textured in this fashion in HeartMates XVE and II have been shown to promote the growth of a stable, adherent biological lining that reduces thromboembolic risk and the level of required anticoagulation therapy.
The electronics and software necessary to control motor drive and levitation are integrated into the implantable motor, and miniaturization was a major focus to make the LVAD as small as possible. Several important design features, including a low-profile apical sewing cuff and quick-connect attachment mechanism, minimize the effective size of the LVAD, and validation studies have shown that the LVAD is readily implanted in the thorax, obviating a pump pocket.
The magnetic levitation technology to achieve the hemocompatibility benefits includes implanted electronics which minimize the number of electrical conductors in the percutaneous cable and stage the LVAD for a future fully implanted configuration, i.e., with no percutaneous lead (see next section). For now, the HeartMate III percutaneous cable has been constructed with an armor layer for damage resistance and features an inline connector that permits a smaller tunneling core and replacement of the external portion without pump replacement if necessary.
The combination of features addressing hemocompatibility (large pump gaps, low shear stress, artificial pulse , and textured blood-contacting surfaces), surgical implantation (small size, engineered apical attachment, and modular driveline), and reduced power consumption is expected to result in an advance in MCS outcomes: lower adverse events and higher patient quality of life .
8.3 Fully Implanted Left Ventricular Assist System
Although there have been HeartMate II patients successfully supported for more than 8 years with a percutaneous lead, it is clear that for maximum freedom from infection and for improved physical and psychological quality of life , a system without percutaneous leads is highly desirable. To meet this need, a fully implanted LVAD system (FILVAS) with transcutaneous energy transmission is in development. We envision a system that would allow a flexible lifestyle with mobile, tethered, and free operation modes (Fig. 8.5) by eliminating the driveline and “around the clock” worn equipment necessary with a percutaneous lead system. Components necessary to eliminate the percutaneous lead include external power conditioner, wearable source coil and internal receiver coil, implanted LVAD controller, and batteries (Fig. 8.6).
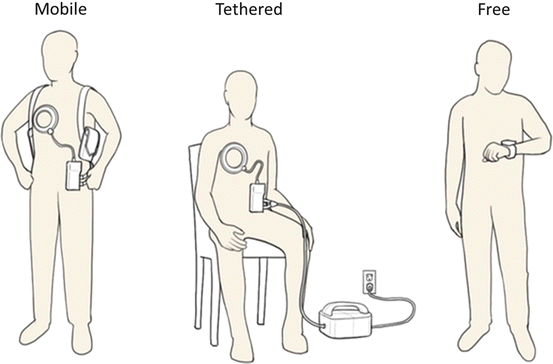
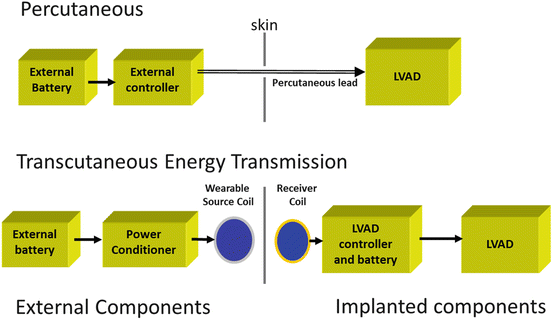

Fig. 8.5
Different configurations for use of the HeartMate fully implanted system (FILVAS). Mobile: Patient in mobile configuration with wearable external batteries and power transmission components for typical mobile use. Tethered: Patient tethered to external power module connected to line power, typically for sleeping and stationary use. Free: Patient in untethered mode with LVAD powered by internal batteries, free from any external equipment. Monitoring of internal device function is achieved with wrist monitor

Fig. 8.6
Comparison of LVAD system with percutaneous lead and with transcutaneous energy transmission. Resonant energy transmission system enables high-efficiency, user-friendly wireless energy transfer across wider distances than with previous designs. Magnetic fields of the two coils couple tightly. AC power through the source coil induces a magnetic field, which induces current in the implanted power capture coil
Previous clinical experience with transcutaneous energy transmission with the AbioCor total artificial heart and the LionHeart left ventricular assist systems [8, 9] provided proof of principle and illustrated the benefits of not having a percutaneous lead. However, these early systems also illustrated several limitations that needed to be overcome. These systems required the external transcutaneous energy transmission (TET) coil to be well aligned and in close proximity with the internal coil for energy transfer. With this close coupling requirement, power transmission was at risk of being disrupted by positional changes or even weight gain. Another limitation was the implanted battery which allowed only a limited amount of tether-free run time, as little as 30 min, and it needed to be replaced too frequently. Major technological advances over the last few years provide the opportunity for fully implanted systems to realize their full potential. These advances include new wireless energy transfer technology and improvements in implanted battery technology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


