Atrial fibrillation (AF) increases by fivefold a patient’s risk for thromboembolic stroke. The main source of emboli in AF is the left atrial appendage (LAA). Therefore, LAA closure could reduce the risk for thromboembolic events in AF. The investigators report the first United States experience with a novel percutaneous LAA closure device, the Lariat snare device, and its outcomes in 21 patients with AF, CHADS 2 scores ≥2, and contraindications to anticoagulation. The LAA was closed with a snare containing suture from within the pericardial space. The intraoperative success of the procedure was confirmed by left atrial angiography and transesophageal echocardiographic color Doppler flow. The effectiveness of the procedure was evaluated by follow-up transesophageal echocardiography. The incidence of periprocedural and short-term complications was assessed by reviewing medical records. Twenty patients (100%) had successful LAA exclusion that was preserved at 96 ± 77 days. No patient had a stroke during an average of 352 ± 143 days of follow-up. One patient had right ventricular perforation and tamponade that required surgical exploration and repair. Two patients required prolonged hospitalization: 1 because of pericardial effusion that required repeat pericardiocentesis and 1 because of noncardiac co-morbidities. Three patients developed pericarditis <1 month after the procedure, of whom 1 had associated pericardial effusion that required drainage. In conclusion, percutaneous LAA exclusion can be achieved successfully and with an acceptable incidence of periprocedural and short-term complications. Further studies are needed to determine whether LAA exclusion lowers the long-term risk for thromboembolic events in patients with AF and contraindications to anticoagulation.
Atrial fibrillation (AF) is estimated to affect >2.3 million patients in the United States alone, a number projected to increase to 5.6 million to 12.1 million by 2050. AF is an independent risk factor for stroke, increasing the risk by fourfold to fivefold. Oral anticoagulation (OAC) with warfarin or newer agents (e.g., dabigatran, rivaroxaban, apixaban) dramatically reduces the risk for stroke in patients with AF. However, all agents approved for stroke prevention in AF are associated with a significant risk for bleeding. A potential strategy for protecting patients with AF from stroke while avoiding bleeding is left atrial appendage (LAA) closure. Blackshear and Odell’s review of data from 23 studies of AF showed that in patients with documented thrombi, 91% of patients (201 of 222) with nonrheumatic AF and 57% of those (254 of 446) with rheumatic AF had left atrial thrombi that were isolated to or had originated from the LAA. Furthermore, a recent study found that LAA closure with an implanted percutaneous device (WATCHMAN; Aritech Inc., Plymouth, Minnesota) was at least as effective as long-term warfarin therapy for preventing stroke. Another percutaneous LAA closure device (Lariat snare device; SentreHEART, Inc., Palo Alto, California), which does not require the placement of intracardiac hardware, has been recently tested in animals. It has also been used in a few patients, but the cases included surgical and percutaneous LAA closures, and no follow-up data were reported. We report our initial experience, including procedural and periprocedural complications and outcomes, with the Lariat snare device in patients with AF with CHADS 2 scores ≥2 and contraindications to or previous failure of OAC.
Methods
Patients with AF (paroxysmal, persistent, or permanent), CHADS 2 scores ≥2, and contraindications to or failure of anticoagulation qualified for percutaneous LAA closure with the Lariat snare device. The device is 510(k) United States Food and Drug Administration approved for soft-tissue approximation and/or ligation with a pretied polyester suture. Common indications for this procedure included a history of severe bleeding, recurrent falls, unexplained persistent anemia, and thromboembolic events despite anticoagulation. Exclusion criteria included previous cardiac surgery, myocardial infarction within the previous 3 months, embolic events within 30 days, New York Heart Association class IV heart failure symptoms, and previous thoracic radiation therapy. Patients were excluded if screening cardiac computed tomography showed the LAA to be superiorly oriented or >50 mm. Most interventions were planned as outpatient procedures to be performed in the cardiac catheterization laboratory with cardiothoracic surgery backup. However, when dictated by either the patient’s clinical status or anticipated difficulties indicated by cardiac computed tomographic findings, the procedure was performed in the hybrid operating room.
All patients gave informed consent to undergo the procedure. Patients were prepared and draped in the usual sterile fashion, and the subxiphoid region was prepped and draped in a sterile fashion for pericardial access. All procedures were performed under general anesthesia. Transesophageal echocardiography (TEE) was performed throughout each procedure. The procedure was performed as previously described in preclinical studies and in a small human feasibility study ( Figure 1 ). Briefly, percutaneous pericardial access was obtained with a technique previously described by Sosa et al. A 17-gauge Tuohy epidural needle (Hakko Corporation, Nagano-ken, Japan) was inserted into the anterior pericardial space, and a 14Fr soft-tipped epicardial guide cannula (SentreHEART, Inc.) was advanced over a 0.035-inch wire. Then, an 8.5Fr SL1 transseptal sheath (St. Jude Medical, St. Paul, Minnesota) was advanced into the left atrium by using standard transseptal puncture techniques. Left atrial angiography was performed in orthogonal views to delineate the LAA ( Figure 1 ). A 0.025-inch guidewire (FindrWIRZ; SentreHEART, Inc.), loaded with an EndoCATH (SentreHEART, Inc.) catheter with a 20-mm balloon at the tip, was positioned in the LAA through the transseptal sheath ( Figure 1 ). A 0.035-inch magnet-tipped epicardial guidewire was inserted through the epicardial guide cannula (Sentre-HEART, Inc.) into the pericardial space and was manipulated until its magnetic tip attached to the 0.025-inch magnetic-tipped endocardial guidewire.
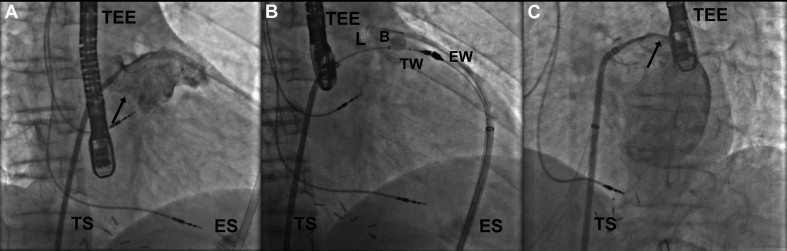
The Lariat snare was advanced over the epicardial magnet-tipped wire and positioned over the proximal part of the LAA, guided by the 20-mm balloon of the EndoCATH catheter inflated with 1:1 normal saline/contrast. Once properly positioned, the snare was closed ( Figure 1 ). After absence of flow from the left atrium to the LAA was confirmed on left atrial angiography and color Doppler TEE, the surgical suture was tightened, the Lariat snare was removed from the pericardial space, and a suture cutter was advanced over the suture to cut it near the LAA ( Figure 1 ). The 14Fr soft-tipped epicardial guide cannula (SentreHEART, Inc.) was exchanged over a 0.035-inch wire for a pericardial drain that was sutured to the skin. From the time of transseptal puncture until the transseptal sheath was removed from the left atrium, patients were anticoagulated with heparin boluses and infusion to a target activated clotting time of >350 seconds. In most cases, heparinization was reversed with protamine at the end of the procedure.
Patients remained hospitalized until the pericardial drain was removed and the absence of significant pericardial fluid was confirmed by transthoracic echocardiography. The LAA exclusion was deemed successful if the LAA Doppler flow was <5 mm on cross section. Each patient was followed up in the clinic by the respective operator (A.M., A.R., H.A.-K., M.S., or M.R.). The procedure time was defined as the time from the pericardial entry to the placement of the pericardial drain.
Data on patient clinical and procedural characteristics and on procedural, periprocedural (to discharge from hospital), and short-term complications (from hospital discharge to 3 months), including stroke, were collected retrospectively from hospital and clinic charts. All transesophageal echocardiograms and transthoracic echocardiograms were read by experienced, board-certified echocardiographers not otherwise involved in the study. Continuous variables are presented as mean ± SD. The present study was approved by our hospital’s institutional review board.
Results
From February 2011 to February 2012, 21 patients (mean age 73 ± 8 years, range 57 to 86; 13 men; mean body mass index 31 ± 7 kg/m 2 ) were selected for percutaneous LAA exclusion ( Table 1 ). Three patients (14%) had paroxysmal AF, 11 (53%) had persistent AF, and 7 (33%) had permanent AF. All patients were at high-risk for stroke; the mean CHADS 2 and CHA 2 DS 2 -VASc scores were 3.2 ± 1.2 and 4.8 ± 1.3, respectively. In fact, 10 patients (48%) had already sustained strokes. In 15 patients (71%), OAC was contraindicated by histories of significant bleeding (gastrointestinal bleeding, hemoptysis, intracerebral hemorrhage, or subdural hematoma). Two patients (10%) had recurrent falls, and the remaining patients had recurrent embolic events despite OAC or difficulty with OAC management. In addition, our patient cohort was at significant risk for bleeding; the mean HAS-BLED score was 3.5 ± 1.0.
| Patient | Gender | Age (yrs) | CHADS 2 Score | CHA 2 DS 2 -VASc Score | HAS-BLED Score | Procedure Time (minutes) | Length of Stay (days) | Time to Follow-Up TEE (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 57 | 5 | 6 | 4 | 129 | 7 | 31 |
| 2 | M | 61 | 2 | 2 | 2 | 58 | 3 | 39 |
| 3 | M | 61 | 5 | 5 | 3 | 99 | 11 | 36 |
| 4 | F | 63 | 3 | 4 | 3 | 57 | 11 | Refused |
| 5 | M | 67 | 2 | 3 | 3 | 105 | 2 | 207 |
| 6 | M | 68 | 2 | 4 | 5 | 87 | 3 | 72 |
| 7 | F | 70 | 3 | 5 | 2 | Excluded | — | — |
| 8 | M | 71 | 2 | 5 | 2 | 97 | 4 | 163 |
| 9 | F | 73 | 2 | 4 | 3 | 62 | 5 | 66 |
| 10 | M | 74 | 3 | 5 | 4 | 82 | 1 | 68 |
| 11 | M | 75 | 3 | 5 | 3 | 80 | 2 | 81 |
| 12 | M | 76 | 3 | 4 | 4 | 86 | 1 | 63 |
| 13 | F | 77 | 4 | 6 | 3 | 75 | 2 | Refused |
| 14 | M | 77 | 3 | 4 | 3 | 105 | 2 | 162 |
| 15 | M | 77 | 5 | 6 | 4 | 75 | 2 | 63 |
| 16 | F | 78 | 3 | 5 | 4 | 95 | 2 | 327 |
| 17 | M | 79 | 2 | 3 | 3 | 48 | 2 | 176 |
| 18 | M | 79 | 3 | 5 | 5 | 76 | 4 | 35 |
| 19 | F | 82 | 6 | 8 | 5 | 62 | 5 | Deceased |
| 20 | F | 84 | 3 | 5 | 3 | 66 | 2 | 82 |
| 21 | F | 86 | 4 | 6 | 5 | 115 | 3 | 72 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


