Fig. 9.1
Neuraminidase and hemagglutinin expression of the influenza virus correlates with epidemic outbreaks
Although its precise provenance is uncertain, the recent epidemic of Novel H1N1 Influenza/2009 started as an outbreak in rural Mexico, where a number of fatal cases were first reported (Dawood et al. 2009). Rapid spread of infection was documented due to air travel, and on June 11, 2009, the World Health Organization (WHO) signaled that a global pandemic of a novel Influenza A (H1N1) was underway by raising the worldwide pandemic alert level to its highest level, Phase 6. The first wave of disease was, in general, mild, with most people recovering without hospitalization; however, excess deaths occurred, particularly in children, otherwise healthy young adults who were immunologically naïve with respect to the virus, diabetics, pregnant women, and immunosuppressed hosts (Table 9.1).
Table 9.1
Risk factors for developing novel H1N1 influenza a
Adults and children over 6 months of age with long-term health conditions |
Chronic lung disease |
Chronic cardiac disease |
Chronic renal disease |
Chronic hepatic disease |
Chronic neurological disease |
Immunosuppression from any cause |
Living in the same household as an immunocompromised patient |
Pregnancy in any stage |
Obesity |
Limited herd immunity due to low vaccination rates |
The severity of disease caused by a novel Influenza virus is difficult to determine with accuracy. Once the disease becomes widespread, the reporting of new cases diminishes so that establishing the prevalence of infection is impossible (Nishiura 2010). Nevertheless, in some countries, the early death rate from novel Influenza A (H1N1) was estimated to be as high as ~10 %. Severe disease was observed in developing regions with low vaccination rates. In patients who required hospitalization, ~25 % required support in an intensive care unit (Jain et al. 2009) (Fig. 9.2). However, the risk and types of bacterial superinfection have been comparable to those of seasonal influenza. Influenza virus is spread by large droplets, small aerosol particles, and contact with fomites (Glezen and Denny 1973; Greenberg 1991; Monto and Sullivan 1993; Whimbey et al. 1996). The incubation period from exposure to viral shedding is 1–3 days, depending on the size of the inoculum. Shedding persists for 5–10 days or longer in immunocompromised hosts and in young children. The clinical features of Influenza A and B virus infection are similar.
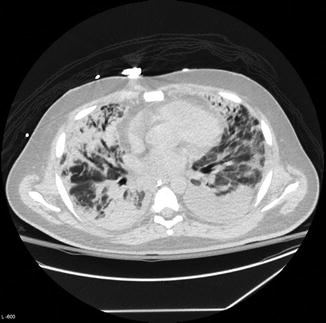

Fig. 9.2
Diffuse pulmonary infiltrates in a patient with novel H1N1 influenza pneumonia (Courtesy S. Digumarthy)
Infections with influenza recently reached epidemic proportions in 2012–2013 in the United States, with reported cases reflecting a mixture of H3N2, H1N1, and influenza B, according to the Center for Disease Control weekly Morbidity and Mortality update. The current influenza vaccine appears to be ~62 % protective but may potentially reduce the severity of disease once contracted. Other concomitant community respiratory infections have complicated the interpretation of vaccine efficacy.
9.4 Ultrastructure
Influenza virus is composed of an RNA core surrounded by a lipid envelope and a virion that appears segmented by ultrastructural analysis (Fig. 9.3). The virion, sometimes circular, while at other times filamentous, contains RNA segments that each include one or two genes. The Influenza A genome contains 11 genes that encode for 11 proteins (Ghedin et al. 2005). The hemagglutinin (HA) and neuraminidase (NA) glycoproteins project as spikes from the surface of the lipid envelope. The HA promotes entry into living host epithelial cells, whereas NA cleaves sialic acid residues, fostering the exit of new virions from the infected cells. The nomenclature for Influenza virus by convention includes the HA and NA specificity; serotype (A, B, or C); geographic origin; strain number; year of isolation; and subtype of virus based on its major antigenic epitopes, e.g., H3N2 (A/Sydney/5/97-like).
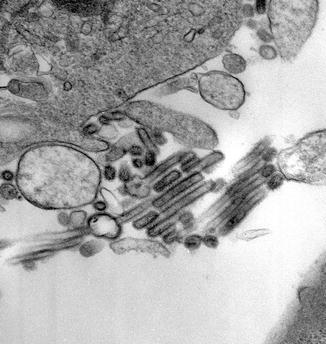

Fig. 9.3
Influenza virus A ×45000 (Courtesy M. Selig)
Once inside the cell, the acidic conditions in the endosome cause the HA of the viral envelope to fuse with vacuolar membranes. An M2 ion channel allows protons to move through the viral envelope, acidifying the core of the virus, causing it both to dissemble and release viral RNA and core proteins. This M2 ion channel is blocked by amantadine drugs (Pinto and Lamb 2006). The core proteins and viral RNA form a complex, which is transported into the cell nucleus, where an RNA-dependent RNA polymerase transcribes complementary positive-sense viral RNA. The viral RNA is next either exported into the cytoplasm for translation or it remains in the nucleus. Newly synthesized viral proteins are secreted through the Golgi apparatus onto the cell surface or transported back into the nucleus, where they can bind viral RNA to form new viral genomic particles.
Hemagglutinin and NA glycoproteins cluster in the cell membrane of the host. New virions budding from the cell acquire a phospholipid membrane coated with the HA and NA embedded in it. Virus adheres to host cells through the HA, and mature virus detaches when the NA cleaves sialic acid residues of the host cell. Oseltamivir inhibits the activities of the NA and prevents the release of new virions. Upon release of influenza virions, the infected host cell generally dies (Cox and Kawaoka 1998).
9.5 Immunology
The mechanisms by which influenza infection causes symptoms in humans have been studied intensively. One of the mechanisms is believed to be the inhibition of adrenocorticotropic hormone (ACTH), resulting in lowered cortisol levels (Jefferies et al. 1998). The viral hemagglutinin protein is responsible for determining where in the human respiratory tract a strain of influenza will bind (Yamada et al. 2006). Strains that are readily transmitted between people have hemagglutinin proteins that bind receptors in the upper part of the respiratory tract. In contrast, the highly lethal H5N1 strain binds to receptors that are mostly found deep in the lungs. This difference in the site of infection may be part of the reason why the H5N1 strain causes severe viral pneumonia in the lungs, but is not easily transmitted by people coughing and sneezing. Common symptoms of the flu such as fever, headaches, and fatigue are the result of the huge amounts of proinflammatory cytokines and chemokines, and this has been proposed to be the cause of the unusual lethality of both the H5N1 avian influenza and the 1918 pandemic strain.
9.6 Clinical Features
The clinical onset of “flu” is abrupt, with prominent systemic symptoms including fever, myalgias, and fatigue. However, symptoms may be limited to rhinorrhea, sore throat, fever, conjunctival injection, and nonproductive cough. The early onset of dyspnea is a poor prognostic sign as it signals progression to respiratory failure.
Acute influenza causes diffuse inflammation of the larynx, trachea, and bronchi, with mucosal injection and edema. Chest pain is a reflection of necrotizing tracheobronchitis. Rates of serious morbidity and death are highest among persons older than 65 years, children aged less than 2 years, and persons of any age who have medical conditions that place them at increased risk for complications from influenza (Whimbey and Bodey 1992; Whimbey et al. 1996; Yousuf et al. 1997). Children are prone to gastrointestinal symptoms, including nausea and vomiting, and febrile seizures are common in young hospitalized children (Bhat et al. 2005). Uncommon complications include myocarditis, pericarditis, myositis, myoglobinuria, encephalopathy, transverse myelitis, and Reye’s syndrome (Glezen and Denny 1973).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


