Our aim was to evaluate the influence of chronic total occlusions (CTOs) on long-term clinical outcomes of patients with coronary heart disease and diabetes mellitus. We evaluated patients with coronary heart disease and diabetes mellitus enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes, who underwent either prompt revascularization (PR) with intensive medical therapy (IMT) or IMT alone according to the presence or absence of CTO. Of 2,368 patients enrolled in the trial, 972 patients (41%) had CTO of coronary arteries. Of those, 482 (41%) and 490 (41%) were in the PR with IMT versus IMT only groups, respectively. In the PR group, patients with CTO were more likely to be selected for the coronary artery bypass grafting stratum (coronary artery bypass grafting 62% vs percutaneous coronary intervention 31%, p <0.001). Compared to the non-CTO group, patients with CTO had more abnormal Q wave, abnormal ST depression, and abnormal T waves. The myocardial jeopardy score was higher in the CTO versus non-CTO group (52 [36 to 69] vs 37 [21 to 53], p <0.001). After adjustment, 5-year mortality rate was significantly higher in the CTO group in the entire cohort (hazard ratio [HR] 1.35, p = 0.013) and in patients with CTO managed with IMT (HR 1.46, p = 0.031). However, the adjusted risk of death was not increased in patients managed with PR (HR 1.26, p = 0.180). In conclusion, CTO of coronary arteries is associated with increased mortality in patients treated medically. However, the presence of a CTO may not increase mortality in patients treated with revascularization. Larger randomized trials are needed to evaluate the effects of revascularization on long-term survival in patients with CTO.
It is unknown whether chronic total occlusions (CTOs) in patients with diabetes mellitus (DM) are associated with worse clinical outcomes and whether revascularization with either coronary artery bypass grafting surgery (CABG) or percutaneous coronary intervention (PCI) is associated with improved all-cause mortality or cardiovascular events. Consequently, we sought to examine the difference in all-cause mortality and major adverse events in patients with diabetes and significant disease with and without CTOs. In addition, we evaluated the difference in survival and adverse events in patients with CTO who received prompt revascularization (PR) with intensive medical therapy (IMT) versus IMT alone according to the extent of coronary artery disease (CAD).
Methods
From January 2001 to March 2005, the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) study enrolled patients with type 2 DM and stable ischemic heart disease. The BARI 2D protocol has been previously published. Briefly, patients were randomized to 2 simultaneous treatment strategies. First, patients were assigned to either PR with IMT versus IMT alone. All patients underwent clinically indicated coronary angiography before randomization. The revascularization strategy was based on clinical and angiographic criteria, procedural safety, expectation of symptom relief, and expected durability of the procedure. The cardiothoracic surgeon strived to bypass all stenoses that were believed to contribute to the patients’ symptoms. Similarly, PCI operators aimed to successfully and safely dilate all significant lesions believed to cause symptoms of ischemia. IMT consisted of 2 simultaneous strategies: pharmacologic therapy and lifestyle modification. Pharmacologic therapy included the administration of antianginal medications, such as β blockers, calcium channel blockers, and long-acting nitrates. In addition, specific risk factor targets were (1) blood pressure ≤130/80 mm Hg, (2) low-density lipoprotein <100 mg/dl, (3) triglycerides <150 mg/dl, and (4) hemoglobin A1c <7.0%. Antianginal medications were further categorized by whether the patient required none, one, or multiple pharmacologic agents for symptomatic control. Strategies for lifestyle modifications include: dietary changes, weight loss, and regular physical exercise as well as smoking cessation. Second, patients were simultaneously assigned to either insulin provision or insulin sensitization therapy to achieve a target glycated hemoglobin level <7.0%. Patients were followed for 6 years or until December 1, 2008. The inclusion criteria were as follows: age 25 or older, type 2 DM, ≥1-vessel CAD (≥50% stenosis) amenable to revascularization, a positive stress test or documented typical angina (with ≥70% stenosis in at least one major epicardial coronary artery), and suitability for PCI or CABG. Patients were excluded if they required immediate revascularization or had left main coronary disease, a creatinine level >2.0 mg/dl, a glycated hemoglobin level >13.0%, heart failure class III or IV, hepatic dysfunction, or previous PCI or CABG within the last 12 months.
CTO was defined as an interruption of blood flow (Thrombolysis in Myocardial Infarction grade 0 flow) of an epicardial coronary artery secondary to complete atherosclerotic luminal stenosis, typically of ≥3 months duration. Angiographic characteristics were assessed at clinical sites. Baseline and index postprocedure angiograms were interpreted by angiographic core laboratory (Stanford University, Palo Alto, California). The revascularization strategy for patients with CTO with PCI or CABG was at the discretion of the treating physician. Patients with CTOs, 3-vessel disease, and proximal left anterior descending artery stenosis were more likely to be treated with CABG. Patients in the PR group were to undergo the procedure within 4 weeks after randomization. Revascularization in the IMT group during follow-up was dictated clinically by the progression of angina or the development of an acute coronary syndrome or severe ischemia. Patients were seen monthly for the first 6 months and then every 3 months thereafter. The primary end point of the study was all-cause mortality. The secondary outcome was a composite of death, myocardial infarction (MI), or stroke.
Continuous variables were presented as medians and interquartile ranges and compared using the Kruskal–Wallis test. Categorical variables were summarized as frequencies and percentages and compared using the chi-square and Fischer exact tests, as appropriate. The main outcome measure was all-cause mortality. The secondary outcome measure was major adverse cardiovascular event (MACE), defined as a composite of death, MI, and stroke. To examine the association between CTO status (patients with vs without CTO) and mortality, Kaplan–Meier curves were fitted; univariate and multivariate Cox proportional hazard ratio (HR) models were used. Observations were censored at the time of occurrence of the outcome or at the end of the study period. Multivariate models were adjusted for patient demographics, serum creatinine, angina within 6 weeks of enrollment, a history of MI, congestive heart failure, hypertension, dyslipidemia, and cerebrovascular accident. Similar methods were used to assess the association between CTO status and secondary outcome of death, MI, or stroke. To assess whether our outcomes of interest differed by treatment status for patients with and without CTO, we further stratified our cohort by the treatment arm in which they were randomized: PR and IMT versus IMT alone. Kaplan–Meier survival curves, univariate, and multivariate Cox proportional hazard models were fitted to each treatment arm group separately. Within each arm, these models tested the association between CTO status and all-cause mortality and MACE. Multivariate analyses were adjusted for patient demographics and co-morbidities pertinent to CAD (see previously). The HRs compare CTO versus non-CTO in patients managed with IMT (i.e., in patients assigned to IMT) and similarly, compare CTO versus non-CTO in patients managed with PR. Furthermore, patients with CTOs were stratified according to the number of lesions with ≥70% diameter stenosis. Within each arm, all-cause mortality and MACE were presented by the number of coronary lesions. Statistical significance was defined as a 2-sided p <0.05. All analyses were performed using SAS, version 9.3 (SAS Institute, Cary, North Carolina) and Stata 11.0 software (Stata Corporation, College Station, Texas). The BARI 2D public use, limited access data set (devoid of personal identifiers) was obtained from the National Heart, Lung, and Blood Institute (National Institutes of Health, Bethesda, Maryland). The Institutional Review Board of the University of Miami Miller School of Medicine (Miami, Florida) then approved the present study.
Results
Of 2,368 patients enrolled in BARI 2D, 972 patients (41%) had a CTO. Three patients had missing data and were excluded from the analysis. There were no significant differences in age, body mass index, dyslipidemia, glycated hemoglobin, or angina between patients with and without CTO. Patients with CTOs and significant disease were more likely to be men, Caucasian, to have previous MI, hypertension, congestive heart failure, and previous revascularization. Patients with CTO compared to patients without CTO had a higher myocardial jeopardy score (52.0 vs 37.0, p <0.001) and were more likely to have abnormal Q waves (24.7% vs 14.9%, p <0.001), abnormal ST depressions (22.7% vs 13.7%, p <0.001), and abnormal T waves (48.6% vs 38.2%, p <0.001; Table 1 ). Patients with CTO were more likely to have abnormal baseline left ventricular systolic function (left ventricular ejection fraction <50%: CTO 229 [24%] vs 171 [13%], p value <0.001).
| Variable ∗ | Early Revascularization (n=1,175) | p -value | Intensive Medical Therapy (n=1,190) | p -value † | ||
|---|---|---|---|---|---|---|
| Total Occlusion (n=482) | No Total Occlusion (n=693) | Total Occlusion (n=490) | No Total Occlusion (n=700) | |||
| Age (years) | 62 [56, 69] | 61 [55, 68] | 0.419 | 63 [56, 68] | 62 [56, 69] | 0.093 |
| Women | 110 (23%) | 238 (34%) | <0.001 | 108 (22%) | 245 (35%) | <0.001 |
| White | 360 (75%) | 436 (68%) | 0.004 | 366 (75%) | 474 (68%) | 0.009 |
| Body Mass Index (kg/m 2 ) | 31 [28, 34] | 31 [27, 35] | 0.894 | 31 [28, 35] | 31 [28, 35] | 0.419 |
| Waist Circumference (cm) | 107 [99,117] | 106 [98, 116] | 0.173 | 107 [99, 116] | 106 [98, 116] | 0.392 |
| Ankle Brachial Index | 1.1 [0.9, 1.2] | 1.1 [0.9, 1.2] | 0.410 | 1.0 [0.9, 1.2] | 1.1 [1.0, 1.2] | 0.009 |
| Hypertension | 387 (81%) | 569 (83%) | 0.311 | 385 (80%) | 585 (84%) | 0.044 |
| Hypercholesterolemia | 400 (84%) | 557 (82%) | 0.210 | 402 (83%) | 552 (80%) | 0.195 |
| Prior Myocardial Infarction | 197 (41%) | 171 (25%) | <0.001 | 214 (44%) | 162 (24%) | <0.001 |
| Prior Congestive Heart Failure | 43 (9%) | 40 (6%) | 0.038 | 36 (7%) | 37 (5%) | 0.147 |
| Prior Cerebrovascular Accident or Transient Ischemic Attack | 46 (10%) | 65 (9%) | 0.925 | 49 (10%) | 70 (10%) | 0.998 |
| Prior Stent | 59 (12%) | 99 (14%) | 0.312 | 56 (11%) | 101 (14%) | 0.132 |
| Prior Revascularization | 124 (26%) | 145 (21%) | 0.054 | 151 (31%) | 137 (20%) | <0.001 |
| Angina equivalent or atypical angina within 6 weeks | 304 (65%) | 447 (65%) | 0.777 | 294 (60%) | 445 (65%) | 0.108 |
| Serum Creatinine (mg/dL) | 1.0 [0.9, 1.2] | 1.0 [0.9, 1.2] | 0.055 | 1.0 [0.9, 1.2] | 1.0 [0.8, 1.2] | 0.018 |
| Hemoglobin-A1C (%) | 7.3 [6.5, 8.6] | 7.1 [6.3, 8.3] | 0.086 | 7.4 [6.5, 8.7] | 7.4 [6.5, 8.5] | 0.861 |
| Myocardial Jeopardy | 54 [37, 71] | 38 [21, 54] | <0.001 | 52 [33, 68] | 36 [21, 53] | <0.001 |
| Abnormal Q wave | 120 (26%) | 94 (14%) | <0.001 | 112 (23%) | 107 (16%) | 0.001 |
| Abnormal ST depression | 95 (23%) | 90 (14%) | <0.001 | 103 (23%) | 84 (13%) | <0.001 |
| Abnormal T Waves | 204 (49%) | 242 (38%) | 0.001 | 219 (48%) | 245 (38%) | 0.001 |
| Medications | ||||||
| Anti-platelet therapy | 145 (21%) | 99 (21%) | 0.882 | 99 (20%) | 118 (17%) | 0.151 |
| Beta Blockers | 364 (76%) | 493 (71%) | 0.088 | 375 (77%) | 485 (70%) | 0.009 |
| Nonsublingual Nitrate | 156 (33%) | 191 (28%) | 0.070 | 160 (33%) | 231 (33%) | 0.847 |
| Diuretics | 198 (41%) | 260 (38%) | 0.222 | 174 (36%) | 279 (40%) | 0.110 |
| Angiotensin Converting Enzyme Inhibitor | 323 (67%) | 425 (62%) | 0.051 | 317 (65%) | 458 (66%) | 0.717 |
| Angiotensin Receptor Blocker | 56 (12%) | 98 (14%) | 0.205 | 69 (14%) | 118 (17%) | 0.185 |
| Aspirin | 426 (89%) | 593 (86%) | 0.097 | 446 (92%) | 605 (87%) | 0.010 |
| Statin | 360 (75%) | 515 (75%) | 0.855 | 384 (78%) | 507 (73%) | 0.030 |
| Insulin | 108 (22%) | 210 (30%) | 0.002 | 125 (26%) | 214 (31%) | 0.058 |
∗ Values are Median [IQR] or n (%).
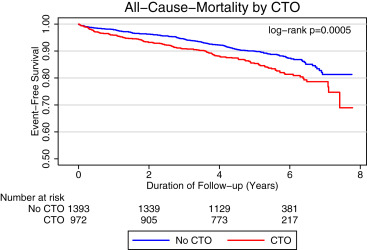
Of patients with CTOs, 482 (41% of total PR group) and 490 (41% of total IMT group) were randomized to PR with IMT and IMT only groups, respectively. Within the PR group, patients with CTO were more often treated with CABG because of greater extent and severity of CAD (CABG in CTO 49% vs CABG in non CTO 21%, p <0.001). Within the PR group, the unadjusted all-cause mortality occurred more frequently in patients with CTO (vs without CTO). Similarly, patients with CTO treated with IMT group had higher all-cause, cardiac, and cardiovascular mortality, compared to patients without CTO. Patients without CTO were more likely to have a subsequent PCI on follow-up ( Table 2 ). Kaplan–Meier survival curve showed an increased risk of all-cause mortality in patients with significant disease and CTO at >5 years of follow-up (HR 1.35 [1.08 to 1.78], p = 0.013; Figure 1 ). Physiological risk factor goals for guidelines contemporaneous with BARI 2D in patients with CTO (vs without CTO) by treatment groups are presented in Table 3 .
| Variable ∗ | Early Revascularization (n=1,175) | p -value | Intensive Medical Therapy (n=1,190) | p -value † | ||
|---|---|---|---|---|---|---|
| Total Occlusion (n=482) | No Total Occlusion (n=693) | Total Occlusion (n=490) | No Total Occlusion (n=700) | |||
| All-cause-mortality | 75 (16%) | 80 (12%) | 0.045 | 82 (17%) | 79 (11%) | 0.007 |
| Cardiac Mortality | 36 (7%) | 36 (5%) | 0.110 | 41 (8%) | 23 (3%) | <0.001 |
| Cardiovascular Mortality | 39 (8%) | 37 (5%) | 0.059 | 45 (9%) | 28 (4%) | <0.001 |
| Major Adverse Cardiovascular Event | 150 (22%) | 116 (24%) | 0.329 | 124 (25%) | 159 (23%) | 0.301 |
| Subsequent Coronary Artery Bypass Surgery | 33 (8%) | 45 (6%) | 0.811 | 91 (19%) | 107 (15%) | 0.134 |
| Subsequent Percutaneous Coronary Intervention | 55 (11%) | 130 (19%) | 0.001 | 99 (20%) | 197 (28%) | 0.002 |
| Fatal or Non-Fatal Myocardial Infarction | 50 (10%) | 78 (11%) | 0.633 | 69 (14%) | 82 (12%) | 0.227 |
| Congestive Heart Failure | 92 (19%) | 100 (14%) | 0.034 | 91 (19%) | 108 (15%) | 0.153 |
| Stroke | 15 (3%) | 17 (3%) | 0.495 | 7 (1%) | 28 (4%) | 0.010 |
| Transient Ischemic Attack | 15 (3%) | 14 (2%) | 0.235 | 10 (2%) | 22 (3%) | 0.247 |
| Unstable Angina Consistent with Acute Coronary Syndrome | 16 (3%) | 19 (3%) | 0.567 | 10 (2%) | 34 (5%) | 0.011 |
| Lower Extremity Revascularization | 12 (2%) | 18 (3%) | 0.908 | 16 (3%) | 23 (3%) | 0.984 |
| Lower Extremity Ulcer | 44 (9%) | 66 (10%) | 0.819 | 36 (7%) | 55 (8%) | 0.744 |
| Severe Hypoglycemia | 25 (5%) | 57 (8%) | 0.044 | 40 (8%) | 54 (8%) | 0.777 |
| Variable ∗ | Early Revascularization | p -value † | Intensive Medical Therapy | p -value † | ||
|---|---|---|---|---|---|---|
| Total Occlusion | No Total Occlusion | Total Occlusion | No Total Occlusion | |||
| Total Number of Visits (n=31,796) | ||||||
| Target Sitting Systolic Blood Pressure < 140 (mmHg) | 75% | 71% | <0.001 | 70% | 68% | 0.014 |
| Low-Density Lipoprotein Cholesterol < 100 (mg/dL) | 59% | 62% | 0.001 | 58% | 58% | 0.736 |
| Hemoglobin A1C < 7 (%) | 43% | 39% | <0.001 | 39% | 40% | 0.183 |
| Regular Exercise | 24% | 28% | <0.001 | 28% | 26% | 0.003 |
| Smoking within One Year | 17% | 14% | <0.001 | 16% | 14% | <0.001 |
| Year 1 (n = 8,576) | ||||||
| Target Sitting Systolic Blood Pressure < 140 (mmHg) | 76% | 70% | <0.001 | 70% | 68% | 0.111 |
| Low-Density Lipoprotein Cholesterol < 100 (mg/dL) | 59% | 61% | 0.303 | 59% | 58% | 0.532 |
| Hemoglobin A1C < 7 (%) | 43% | 39% | 0.018 | 38% | 40% | 0.220 |
| Regular Exercise | 24% | 27% | 0.015 | 28% | 25% | 0.040 |
| Smoking within One Year | 13% | 12% | 0.106 | 12% | 14% | 0.089 |
| Year 2 (n = 8,054) | ||||||
| Target Sitting Systolic Blood Pressure < 140 (mmHg) | 76% | 71% | 0.001 | 70% | 68% | 0.176 |
| Low-Density Lipoprotein Cholesterol < 100 (mg/dL) | 60% | 61% | 0.757 | 58% | 59% | 0.661 |
| Hemoglobin A1C < 7 (%) | 43% | 39% | 0.006 | 38% | 40% | 0.298 |
| Regular exercise | 24% | 28% | 0.022 | 28% | 26% | 0.178 |
| Smoking within One Year | 10% | 12% | 0.104 | 11% | 13% | 0.031 |
| Year 3 (n = 7,171) | ||||||
| Target Sitting Systolic Blood Pressure < 140 (mmHg) | 75% | 71% | 0.005 | 70% | 70% | 0.606 |
| Low-Density Lipoprotein Cholesterol < 100 (mg/dL) | 60% | 62% | 0.205 | 58% | 59% | 0.622 |
| Hemoglobin A1C < 7 (%) | 44% | 38% | 0.001 | 38% | 39% | 0.335 |
| Regular Exercise | 25% | 27% | 0.127 | 27% | 26% | 0.763 |
| Smoking within One Year | 11% | 9% | 0.091 | 11% | 11% | 0.537 |
| Year 4 (n = 4,847) | ||||||
| Target Sitting Systolic Blood Pressure < 140 (mmHg) | 74% | 71% | 0.151 | 70% | 70% | 0.915 |
| Low-Density Lipoprotein Cholesterol < 100 (mg/dL) | 59% | 62% | 0.123 | 57% | 59% | 0.519 |
| Hemoglobin A1C < 7 (%) | 44% | 39% | 0.008 | 42% | 40% | 0.322 |
| Regular exercise | 26% | 30% | 0.069 | 28% | 28% | 0.850 |
| Smoking within One Year | 13% | 9% | 0.007 | 10% | 9% | 0.743 |
| Year 5 (n = 2,595) | ||||||
| Target Sitting Systolic Blood Pressure < 140 (mmHg) | 72% | 75% | 0.222 | 69% | 72% | 0.226 |
| Low-Density Lipoprotein Cholesterol < 100 (mg/dL) | 59% | 68% | 0.001 | 55% | 59% | 0.114 |
| HbA1C < 7% | 44% | 38% | 0.042 | 47% | 42% | 0.119 |
| Regular Exercise | 26% | 31% | 0.035 | 26% | 29% | 0.245 |
| Smoking within One Year | 13% | 11% | 0.179 | 10% | 12% | 0.386 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


