Normal aging results in a predictable decrease in glomerular filtration rate (GFR), and low GFR is associated with worsened survival. If this survival disadvantage is directly caused by the low GFR, as opposed to the disease causing the low GFR, the risk should be similar regardless of the underlying mechanism. Our objective was to determine if age-related decreases in estimated GFR (eGFR) carry the same prognostic importance as disease-attributable losses in patients with ventricular dysfunction. We analyzed the Studies Of Left Ventricular Dysfunction limited data set (n = 6,337). The primary analysis focused on determining if the eGFR-mortality relation differed by the extent to which the eGFR was consistent with normal aging. Mean eGFR was 65.7 ml/min/1.73 m 2 (SD = 19.0). Across the range of age in the population (27 to 80 years), baseline eGFR decreased by 0.67 ml/min/1.73 m 2 /year (95% confidence interval [CI] 0.63 to 0.71). The risk of death associated with eGFR was strongly modified by the degree to which the low eGFR could be explained by aging (p for interaction <0.0001). For example, in a model incorporating the interaction, uncorrected eGFR was no longer significantly related to mortality (adjusted hazard ratio 1.0 per 10 ml/min/1.73 m 2 , 95% CI 0.97 to 1.1, p = 0.53), whereas a disease-attributable decrease in eGFR above the median carried significant risk (adjusted hazard ratio 2.8, 95% CI 1.6 to 4.7, p <0.001). In conclusion, in the setting of left ventricular dysfunction, renal dysfunction attributable to normal aging had a limited risk for mortality, suggesting that the mechanism underlying renal dysfunction is critical in determining prognosis.
Renal dysfunction (RD) has emerged as one of the strongest prognostic indicators in patients with heart failure (HF). However, it is unclear if these poor outcomes are the direct result of adverse effects of a reduction in glomerular filtration rate (GFR) or if the RD is primarily a surrogate for greater HF disease severity. There are multiple theoretical mechanisms that would implicate the decrease in GFR itself as a primary culprit mechanism. However, recent human observational data have suggested that not all decreases in renal function are prognostically equivalent. An accessible approach toward this question of causality may be studying the effect of “normal” aging. After a peak in GFR in the third decade of life of approximately 130 ml/min/1.73 m 2 , renal function progressively decreases on the order of 0.8 ml/min/1.73 m 2 /year. Notably, in studies of the general population, the relative risk for death associated with any given estimated GFR (eGFR) progressively diminishes as age increases. Although believed to be part of the “normal” aging process, the resultant reduction in GFR that occurs with aging is a true loss of GFR and physiologically indistinguishable to that produced by pathologic reductions in GFR. As a result, if the adverse outcomes associated with RD stem from the reduction in GFR, it should be irrelevant whether the low GFR is the result of a “benign” cause such as the aging process or a pathologic process such as HF-induced RD. However, if RD is primarily acting as an indicator of otherwise unmeasured disease severity, the risk for mortality attributable to RD should be much greater in patients who have a reduction in GFR primarily as a result of a pathologic cause. As such, the focus of the study was determining if the eGFR-mortality relation differed by the degree to which low eGFR was driven by normal aging.
Methods
The Studies Of Left Ventricular Dysfunction (SOLVD) prevention and treatment trials were National Heart, Lung and Blood Institute–sponsored, randomized, double-blind, placebo-controlled trials of the effect of enalapril in patients with asymptomatic and symptomatic left ventricular dysfunctions and comprise the overall SOLVD population. Methods and results have been previously published. Briefly, 4,228 patients were enrolled in the prevention trial and 2,569 patients in the treatment trial at 23 international centers (total n = 6,797). Inclusion in either trial required an ejection fraction of ≤35% and age from 21 to 80 years. Exclusion criteria included a baseline creatinine level of >2.5 mg/dl. Patients with data available to estimate baseline GFR (n = 6,782) were included in the present analysis.
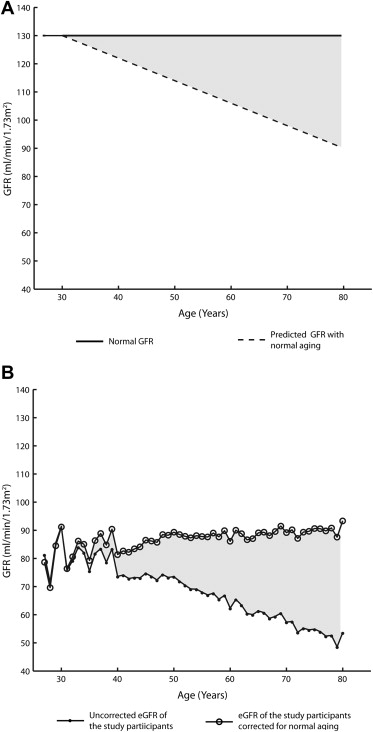
Estimated GFR, uncorrected for aging, was calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation. The normal GFR that would be expected with healthy aging was calculated by the equation {age-predicted GFR = 130 ml/min/1.73 m 2 − [(age in years − 30) × 0.8]}, given that normal GFR in adults before the age of 30 years is approximately 130 ml/min/1.73 m 2 after which time it is reported to decrease on the order of 8 ml/min/1.73 m 2 per decade. Given that HF is not known to protect against age-related decreases in renal function, to account for normal decrease that would be expected in the population, an age-corrected eGFR was calculated by the equation {age-corrected eGFR = MDRD eGFR + [(age in years − 30) × 0.8]}. Disease-attributable decrease in eGFR (i.e., the decrease in eGFR beyond that expected for normal aging) was calculated by subtracting the age-corrected eGFR from 130 ml/min/1.73 m 2 . The same parameter could be calculated by subtracting the eGFR from the age-predicted GFR. The entire SOLVD population was analyzed as a whole to maximize power given the lack of interaction across the prevention versus treatment trials in the primary analysis (3-way interaction p = 0.38).
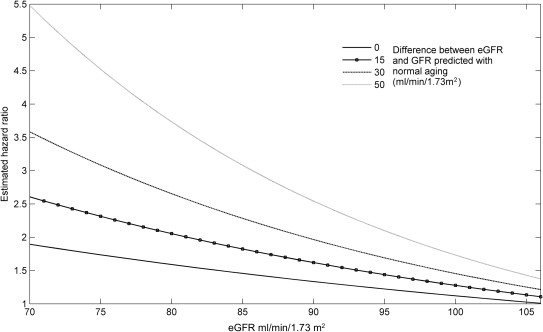
The primary analyses in this study focused on determining if the risk for death associated with eGFR was modified by the degree to which the low eGFR was the result of disease versus normal aging. As such, the primary end point was the interaction among eGFR, the disease-attributable decrease in eGFR, and mortality. Values reported are mean ± SD, median (quartile 1 to quartile 4), and percentile. Independent Student t test or the Mann-Whitney U test was used to compare continuous parameters. Pearson chi-square test was used to evaluate categorical variables. Linear regression was used to estimate the decrease in renal function across the age range of the population. Proportional hazards modeling was used to evaluate time to event associations with all-cause mortality. To determine if effect modification was present between disease-attributable decrease in eGFR and the eGFR-mortality association, a model incorporating the main effect of eGFR, the main effect of disease-attributable decrease in eGFR, and the 2-way interaction of these terms was constructed. Candidate covariates for multivariate modeling were obtained by screening all baseline variables for a univariate association with mortality (p ≤0.2). Covariates were removed using backward elimination (likelihood ratio test), and variables with p <0.2 were retained. Loess approach with span 5 was used to estimate the trends of eGFR and age-corrected eGFR ( Figure 1 ). Using a weighted proportional hazards model of overall survival with independent variables such as eGFR, disease-attributable decrease in eGFR, and their interaction, we estimated the hazard ratio with respect to eGFR as 106.5 and with zero disease-attributable decrease in eGFR. A reference value of 106.5 ml/min/1.73 m 2 was used as this is the median age-predicted GFR in the population. Specifically, the hazard ratio at a value of (eGFR, disease-attributable decrease in eGFR) in Figure 2 is calculated as the hazard rate at the value of (eGFR, disease-attributable decrease in eGFR) divided by the hazard rate at (eGFR, disease-attributable decrease in eGFR) = (106.5, 0). Significance was defined as 2-tailed p <0.05 for all analyses excluding tests of interaction in which p values <0.1 were considered significant. Statistical analysis was performed with IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, New York), Statistical Analysis System (SAS), version 9.3 (SAS Institute, Cary, North Carolina), and Matrix Laboratory (MATLAB), version R2012 (The MathWorks, Inc., Natick, Massachusetts).
Results
Baseline characteristics of patients in this analysis are presented in Table 1 . The age of the population ranged from 27 to 80 years with a mean of 59 ± 10 years. The mean eGFR was 65.7 ml/min/1.73 m 2 (SD = 19.0), which is 51.0% (41.3% to 59.2%) below a truly normal GFR of 130 ml/min/1.73 m 2 ( Figure 1 ). In the overall population, 35.1% (27.2% to 43.6%) of this deviation from true normal was consistent with expected age-related loss, and 62.9% (54.4% to 70.3%) was consistent with a disease-attributable decrease in eGFR. However, this differed across the age range of the population where the absolute degree of disease-attributable decrease in eGFR remained relatively stable, but progressive age-related loss accrued over time ( Figure 1 ). Interestingly, the loss in eGFR with progressive age in the population was not greater than that described in healthy populations ( shaded areas in Figure 1 ) as eGFR decreased linearly at a rate 0.67 ml/min/1.73 m 2 /year (95% confidence interval [CI] 0.63 to 0.71). Consistent with the above, correction of eGFR for the reported age-related decrease in eGFR found in other populations (0.8 ml/min/1.73 m 2 /year over the age of 30 years) resulted in a relatively stable baseline eGFR across the range of age in the population ( Figure 1 ).
| Characteristic | Value, n = 6,782 (%) |
|---|---|
| Age (yrs) | 59.3 ± 10.2 |
| White | 5,982 (88) |
| Men | 5,866 (86) |
| Hypertension | 2,645 (39) |
| Diabetes mellitus | 1,309 (19) |
| Coronary heart disease | 5,066 (75) |
| Heart rate (beats/min) | 76 ± 12 |
| Systolic blood pressure (mm Hg) | 119 ± 17 |
| Enalapril | 3,391 (50) |
| β Blocker | 1,207 (18) |
| Digoxin | 2,245 (33) |
| Loop diuretic | 2,204 (33) |
| Serum sodium (mmol/L) | 140 ± 3 |
| Creatinine (mg/dl) | 1.2 ± 0.3 |
| Uncorrected eGFR (ml/min/1.73 m 2 ) | 66 ± 19 |
| eGFR corrected for normal aging (ml/min/1.73 m 2 ) | 88 ± 17 |
| Disease-attributable decrease in eGFR (ml/min/1.73 m 2 ) | 42 ± 17 |
| Left ventricular ejection fraction (%) | 27 ± 6 |
| New York Heart Association class | 1.7 ± 0.7 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


