Fig. 5.1
The dorsal mesocardium and development of the dorsal mesenchymal protrusion. Panels a and b show the dorsal mesocardium at the venous pole of an E9.5 mouse heart. The pulmonary vein is situated in the midline of this structure. Panels c and d are cartoons depicting the venous pole of the mouse heart at E9.5 (c) and E11 (d), illustrating how the dorsal mesenchymal protrusion develops between the right dorsal mesocardial reflection and the developing pulmonary vein. Panel e shows a section of a human embryonic heart during the 7th week of development, immunostained for atrial myosin heavy chain [10], demonstrating the wedged position of the DMP and the displacement of the mouth of the central pulmonary vein into the left atrium. Abbreviations: DM dorsal mesocardium, DMP dorsal mesenchymal protrusion, LA left atrium, LSH left sinus horn, PAS primary atrial septum, PuV pulmonary vein, RSVC right superior vena cava, RA right atrium, RVV right venous valve (Adapted from: [11])
5.3.4 The Sinus Venosus
As described in the introduction, after the initial formation of the tubular heart, tissues subsequently are added at the arterial and venous pole during looping. During this process the sinus venosus is formed. Once formed, the sinus venosus consists of a left and right sinus horn and can be seen as a prominent structure in the developing human heart [15], receiving the venous return from the left and right anterior and posterior cardinal veins. As development progresses, the sinus venosus becomes largely incorporated into the back wall of the right atrium, resulting in the formation of the sinus venarum. This process also leads to the incorporation of the orifices of the superior and inferior caval veins, derivatives of the right-sided cardinal veins, and the orifice of the coronary sinus into the right atrium. In the human embryo, the connections of the left-sided cardinal vein and other-left sided venous structures associated with the left horn of the sinus venosus normally regress. This process is responsible for the formation of the coronary sinus. Thus, in the normal human heart, the coronary sinus is the derivative of the left horn of the sinus venosus. Failure of the left sinus horn to regress leads to persistence of the left superior caval vein. It is noteworthy that this regression of the left sinus horn does not occur in the mouse and that a left superior caval vein is part of the normal murine cardiovascular anatomy.
5.3.5 The Dorsal Mesenchymal Protrusion
The atrial septal complex functionally separates the left from the right atrium. In recent years it has become increasingly clear that atrial septation is intrinsically associated with the development of the tissues at the atrioventricular junction [8, 11]. Incomplete formation of elements of the atrioventricular septal complex results in atrial and atrioventricular septal defects [16–19]. The dorsal mesenchymal protrusion (DMP, also known as the vestibular spine) plays a critical role in atrial and atrioventricular septation. The mesenchymal structure that we now know as the DMP was first described in detail in studies of the developing human heart [15]. In these studies it was reported that the mesenchyme of the DMP was immunohistochemically distinct from the endocardially derived mesenchyme of the atrioventricular cushions, suggesting a different origin. With the development of transgenic mouse technology that enabled developmental biologists to perform cell fate studies, it was determined that the mesenchyme of the DMP, indeed, is not an endocardially derived tissue [8, 20] but instead a derivative of the SHF [16–18]. During its development, the DMP extends ventrally into the common atrium using the dorsal mesocardium as its portal of entry (Fig. 5.1c, d). From the earliest stages at which it can be recognized, the DMP is continuous with two endocardially derived cell populations: the mesenchymal cap that extends along the leading edge of the septum primum and the inferior AV cushion. Whereas the DMP initially develops as a mesenchymal tissue, after completion of atrial septation, the SHF-derived mesenchyme of the DMP eventually undergoes a mesenchymal-to-myocardial differentiation, characterized by a decrease in the expression of islet 1 (Isl1) and an increase in NK2 homeobox 5 (Nkx2-5) in DMP-derived cells. This myocardialized DMP becomes the muscular inferior rim at the base of the oval fossa [18]. In the setting of ostium primum defect, a structural heart defect that allows shunting between the left and right atrium and a common abnormality found in all patients with atrioventricular septal defects (AVSDs), this DMP-derived structure is typically missing [14].
5.3.6 The Components of the Atrial Septum
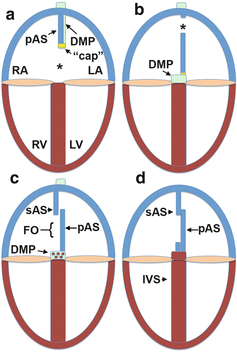
Atrial septation comprises of a series of complicated events that can be summarized (and simplified) as follows. It all starts with a common atrium in which no atrial septal structure can be identified. The first event in the septation process is the development of the primary atrial septum (septum primum). The base of the primary atrial septum develops in close association with the left mesocardial reflection of the dorsal mesocardium and grows from the dorsal wall of the common atrium (Fig. 5.1e). As mentioned above, a mesenchymal “cap” is located on the leading edge of the primary atrial septum which is contiguous with the developing DMP. The left and right atria at this stage communicate with each other through the opening under the primary atrial septum, the primary interatrial foramen (ostium primum) (Fig. 5.2a). As the mesenchymal tissues of the major AV cushions, the mesenchymal “cap,” and the DMP fuse and close the primary foramen [8], a secondary interatrial foramen (ostium secundum) forms in the body of the primary septum, allowing continuous shunting of blood from the right to left atrium, critical for the embryonic circulation (Fig. 5.2b). This step is followed by the development of the secondary atrial septum (septum secundum). As the secondary septum grows in, it descends into the atrial cavity to cover the secondary foramen (Fig. 5.2c). The mechanism by which the secondary septum develops appears different between man and mice. In the human heart, the secondary septum primarily develops by infolding of the atrial roof, whereas in the mouse active myocardial outgrowth forms the base of its development. After its formation, the secondary septum overlaps with the upper part of the primary atrial septum. As a result, the primary atrial septum basically becomes a temporary one-way valve allowing shunting from right to left but limiting/preventing left-to-right shunting. In the majority of the human population, the secondary and the primary septum eventually fuse, sealing off the potential of left-right shunting at the atrial level (Fig. 5.2d). However, in a significant percentage (25 %) of human individuals, the two septa will not fuse, resulting in a condition known as patent foramen ovale (PFO).