Fig. 13.1
Critical limb ischaemia with forefoot gangrene
Inflammatory markers have been evaluated among both asymptomatic [31–33] and symptomatic [31–34] subjects with PAD, however (Table 13.1). Furthermore, such markers have also been shown to be related to the future development of PAD and CLI already in subjects without detectable disease. For example, being in the highest quartile of hs-CRP among healthy subjects increases the risk for the development of PAD by a factor of 2.8 [22], and elevated levels of proteins such as fibrinogen, alpha 1-antitrypsin, haptoglobin, ceruloplasmin, and orosomucoid measured in 5,619 healthy men without symptoms suggestive of PAD have been associated with increased risk for the development of PAD requiring revascularization during 16 years of follow-up, even after adjustment for other relevant risk factors [35]. Multisite atherosclerotic disease is also reflected in more intensive inflammatory activity; among 234 patients undergoing coronary angiography, Brevetti and co-workers found that levels of both hs-CRP and IL-6 were higher in patients with both CAD and PAD or CAD alone or than in control subjects without either disease. Furthermore, hs-CRP was higher in patients with both diseases than in those with only CAD [34], indicating a more active inflammatory process in multisite disease.
Table 13.1
Some different markers and mediators of inflammation associated with peripheral arterial disease (PAD) and critical limb ischemia (CLI) in different studies
Variable | Feature of PAD | References |
|---|---|---|
α1-Antitrypsin | PAD development | [35] |
α-Defensin | PAD severity, cardiovascular mortality in PAD | [52] |
Amyloid A | Adverse prognosis after intervention for PAD | [43] |
Ceruloplasmin | PAD development | [35] |
Fibrinogen | PAD development, mortality in CLI | |
Haptoglobin | PAD development | [35] |
High sensitivity C-reactive protein | PAD development, occurrence, progression, and severity, adverse prognosis after intervention for PAD, cardiovascular mortality in PAD, mortality in CLI | |
Interleukin-6 | PAD occurrence and progression, mortality in CLI | |
8-Isoprostane-PGF2α | PAD occurrence | [36] |
Leukocyte count | PAD occurrence, mortality in CLI | [54] |
Matrix metalloproteinases 2 and 9 | PAD and CLI development | |
Neopterin | Mortality in CLI | [53] |
Neutrophil/lymphocyte ratio | Mortality in CLI | [54] |
Orosomucoid | PAD development | [35] |
Tumor necrosis factor-α | Mortality in CLI | [53] |
Oxidative stress, as reflected by increased levels of 8-iso-PGF2α, has also been related to PAD. In a study of 100 patients with PAD and 100 control subjects without clinically relevant atherosclerotic disease who were all non-smokers and not taking any lipid-lowering drugs or vitamins to avoid possible effects on isoprostanes, 8-iso-PGF2α levels were 1.5-fold higher among PAD patients than in controls [36]. Symptomatic PAD is also associated with increased levels of both IL-6 and hs-CRP compared with subjects without PAD, even after adjustment for BMI, smoking, and cholesterol [37]. In vitro culturing of whole blood and profiling of the production of several different interleukins and other cytokines confirmed this inflammatory hyperresponsiveness related to associated leukocytosis in PAD patients [38]. Matrix metalloproteinases have also been evaluated in relation to PAD, and increased plasma levels of both MMP-2 and MMP-9 were found to be correlated with development of both PAD and CLI [39]. Genetic studies have also confirmed the above-mentioned connections between inflammatory markers and CLI. Gene polymorphisms related to several markers for inflammation, both cytokines and matrix metalloproteinases, IL-6, E-selectin, ICAM-1, MCP-1, MMP-1, and MMP-3, are all independently associated with PAD, and the different risks for PAD and CLI depend on the number of high-risk genotypes concomitantly carried by the individual subject [40]. Furthermore, the IL-6 gene is upregulated in the hypoperfused musculature of CLI patients [41], corroborating the above-mentioned observation that IL-6 has been found to be increased in symptomatic peripheral artery disease [31]. It is important to note that all study results have not shown uniform results; however, in a small group of eight CLI patients including five with ischemic lesions [42] neither TNF-α nor IL-6 differed from values in healthy controls.
Inflammatory Markers During Invasive Treatment of PAD and CLI
Potential relationships between inflammatory markers and invasive treatment of PAD and CLI have also been evaluated (Table 13.1). Different markers such as high sensitivity (hs)-CRP, fibrinogen, and serum amyloid A (SAA) measured preoperatively in patients planned for lower extremity bypass all correlate with an increased risk for later adverse graft-related or cardiovascular events [43]. For hs-CRP, this relationship persisted even in multivariable analysis. In the post-treatment period after both open and endovascular repair in different vascular segments, several of the above-mentioned platelet and leukocyte mechanisms are further activated. Such post-interventional patterns have also mainly been investigated after interventions in coronary vessels, however. For example, levels of P-selectin [44] mediating platelet-leukocyte binding [45] are increased, and leukocyte-platelet interactions are increased after percutaneous coronary interventions [46]. In coronary blood, both expression of the activated platelet fibrinogen receptor [47] and release of chemoattractants affecting neutrophils [48] increase after endovascular coronary interventions. Reports on patterns of inflammatory substances during and after invasive vascular interventions for PAD are scarcer. In an observational study with repeated arterial sampling from 14 patients undergoing angiography for aortoiliac atherosclerotic disease, 9 of whom underwent PTA, we could not demonstrate any definite signs of leukocyte activation during or immediately after peripheral angiography [49]. The levels of inflammatory mediators after peripheral vascular interventions are not clinically irrelevant, however, and the occurrence of restenosis after vascular injury, such as for example an episode of invasive treatment, also has inflammatory features. A postischemic macrophage activation state evaluated in an animal model has been suggested as a new potential therapeutic approach to protect tissues from necrosis and promote tissue repair during CLI [50]. As no definite relationships have been reported between levels of inflammatory mediators and the efficacy of interventions, inflammatory mediators cannot yet be used to determine the best treatment of a certain patient.
Inflammatory Markers, Prognosis, and Mortality in Critical Lower Limb Ischemia
Even if the prognostic importance of inflammatory activation is less well established in PAD and CLI than in patients with coronary or precerebral atherosclerosis, some observations about the relevance of inflammatory activation for prognosis have been presented also in PAD patients (Table 13.1). Elevated levels of inflammatory mediators seem to be indicators of a more severe prognosis concerning both local progression of atherosclerosis in the affected limb and acute events occurring in other vascular territories. The first mechanism is exemplified by the fact that both CRP and IL-6 levels predict local progression of atherosclerosis in the lower limb during 12 year follow-up of the ankle-brachial index (ABI) [51].
A recent investigation showed that patients with CLI show significantly higher α-defensin and hs-CRP levels compared with patients with intermittent claudication (IC) [52]. Furthermore, within the IC group high concentrations of α-defensin and high hs-CRP conferred a five times higher risk for cardiovascular mortality during follow-up than in patients with either high α-defensin or high hs-CRP. The addition of α-defensin or hs-CRP to conventional risk factors thus improved risk prediction concerning cardiovascular mortality in patients with this manifestation of PAD [53].
The high 1-year mortality of around 20–25 % in patients with CLI [7, 21, 22, 52] is mainly due to cardiovascular disease. Furthermore, this already high mortality rate is increased even more in patients with features of inflammation, such as increased leukocyte count and fibrinogen level [23], or an elevated neutrophil/lymphocyte ratio and increased troponin levels [54]. The inflammatory mediators IL-6, TNFα, neopterin, and hs-CRP have also been associated with 1-year mortality in subjects with CLI [53]. For TNFα and neopterin, this association was independent of other variables, such as age, sex, gangrene, lipid-lowering therapy, leukocyte count, renal function, and HDL cholesterol. As the relationships between inflammatory mediators and mortality persisted after exclusion of patients with gangrene, these relationships may only partly be explained by the fact that patients with inflammatory processes such as gangrene of the extremities showed a high mortality [53]. Furthermore, when the patient material was later analyzed concerning 5- and 10-year mortality, the predictive value of TNFα at diagnosis persisted (Fig. 13.2). Data are partly conflicting, however, as no associations were found among α-defensin, hs-CRP, and mortality in CLI patients in the above-mentioned study by Urbonaviciene and co-workers [52].
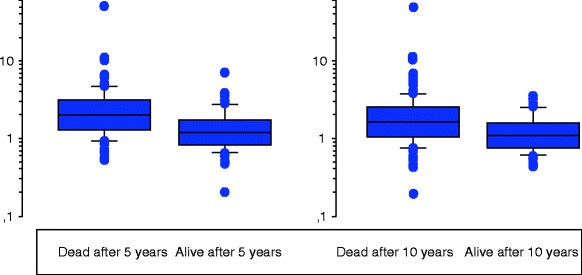

Fig. 13.2
Tumor necrosis factor-α (pg/ml, y-axis) at diagnosis in 259 patients [53] with critical limb ischaemia in relationship to 5- (left panel, p < 0.001) and 10- (right panel, p < 0.001) year survival
Conclusions
In conclusion, low-grade inflammatory activation predicts the development of PAD already in healthy subjects. The degree of such inflammatory activation is related to both the severity and progression of disease and to cardiovascular mortality in PAD patients. No definite relationships have yet been reported between levels of inflammatory mediators and the efficacy of interventions, and inflammatory mediators cannot yet be used to determine the best treatment for the individual patient.
References
2.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43.PubMedCrossRef
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


