Chapter 79 Inflammatory Bowel Disease
Epidemiology
The true incidence of thoracic complications in IBD patients is unknown. There have been no screening tests performed on large populations of IBD patients. The proportion of IBD patients with subjective respiratory symptoms such as cough, sputum production, wheezing, or shortness of breath has been reported to be as high as 50%. One or more pulmonary function tests (PFTs) are abnormal in approximately 40% of IBD patients, with forced expiratory volume in 1 second (FEV1), inspiratory vital capacity (IVC), or diffusion capacity (DLCO) showing a 10% to 30% reduction. Between 25% and 50% of asymptomatic IBD patients show abnormalities on high-resolution computed tomography (HRCT) scans. Often, the findings are subtle and include ground-glass opacities, mosaicism suggestive of air trapping, peripheral opacities, and cysts. At the other end of the spectrum are case reports and small series of patients with distinctive manifestations of thoracic involvement. These reports, accounting for 155 patients, were recently reviewed (see Suggested Readings). Although nothing is offered to calculate incidence or prevalence, significant symptomatic thoracic involvement by IBD probably is not a common event. However, it is common enough that physicians need to be cognizant of IBD to recognize it and prevent the potentially debilitating consequences of some of its forms.
Clinical and Pathologic Features
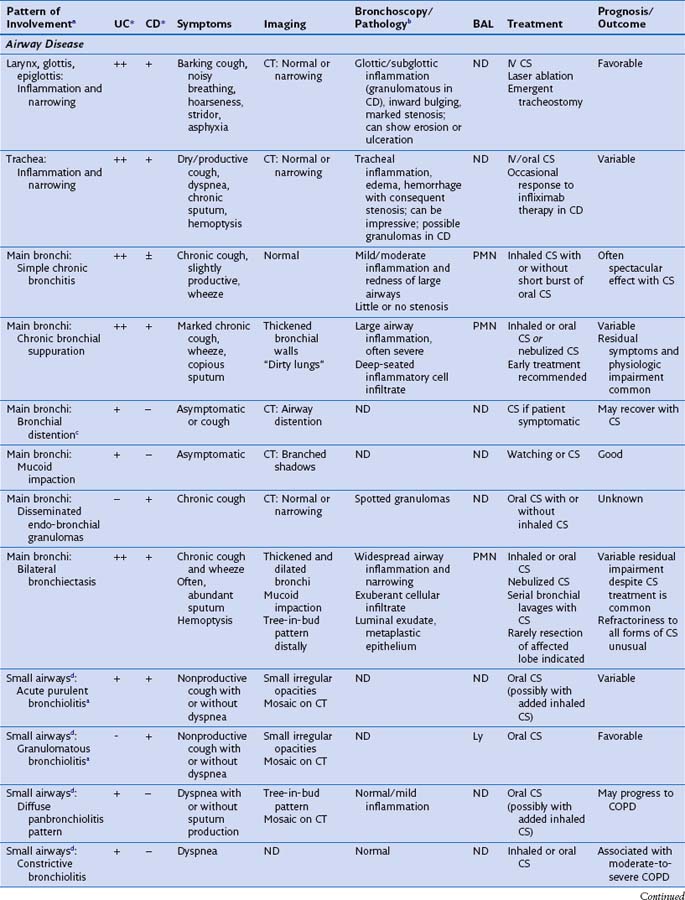
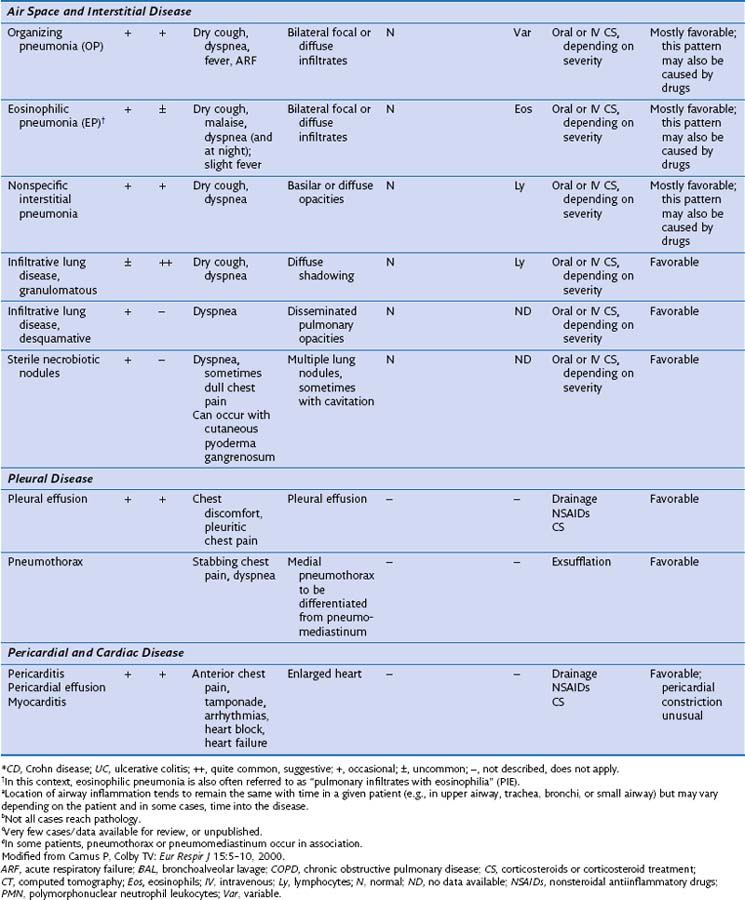
This section discusses the different compartments of the respiratory tract involved by IBD, including unique considerations of the particular location, clinical presentation, and its pathologic features (Table 79-1).
Airway Disease
Radiographically, airway walls appear thickened with a “tram line” pattern. Larger airways show mucus plugging, smaller airways a tree-in-bud appearance. These changes tend to occur in a basilar-predominant distribution, occasionally with associated volume loss. Small airways disease can be associated with a tree-in-bud pattern, diffuse reticular shadows, or mosaic pattern due to air trapping (Figure 79-1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree