Fig. 1.1
Global cardiovascular/cardiometabolic risk factors (RFs), including the established Framingham Risk Score RFs and the emergent metabolic syndrome RFs related with obesity and inflammation
Established vs. Emergent Biomarkers: Experimental and Clinical Data
Biomarkers and/or risk factors can be either biochemical, physiological, anatomical, or physical, and they can be classified into three broad categories, genetic (including tissue or cellular) biomarkers, imaging biomarkers, and circulating biomarkers, and further into traditional and nontraditional (novel or emergent) risk factors or biomarkers.
The distinction between risk factors and biomarkers for a disease is subtle. The traditional view considers inflammatory biomarkers as risk markers rather than risk factors. A risk factor has a biological role in disease, while a biomarker that is also a risk factor is able to measure a step in pathological processes. A risk marker is statistically associated with the disease, but causality is not an obligatory characteristic of the marker: it is an indirect measure of disease processes and may be a response to other risk factors indeed involved in the pathogenesis, as was previously revised [23, 24].
During the last decades, advances in biomarker research and discovery have allowed remarkable progress in diagnosis and management of several diseases. The US Food and Drug Administration (FDA) defined biomarker as a substance that can be objectively measured as an indicator of normal biologic or pathogenic processes or of pharmacologic responses to therapeutic intervention [25]. In fact, biomarkers provide a more direct measure of a disease pathway and are ubiquitous tools that can aid in understanding disease mechanisms as well as in predicting, diagnosing, and monitoring disease processes.
CVD are caused by a number of potentially modifiable etiologies and risk factors and have been one of the major areas of biomarker research and application, especially because CHD remains the leading cause of morbidity and mortality in the developed world. Biomarkers are crucial for the quantitation of this risk. The concept of global risk assessment was introduced by the Framingham Heart Study more than 50 years ago; however, atherosclerosis is now clearly recognized as having an inflammatory signature, recommending upgrade of risk prediction tools. Understanding the inflammatory cascade in the development of atherosclerosis allows the consideration of a number of inflammatory markers as potentially useful predictors of CVD. Although there is no debate that the inflammatory process is essential to the atherosclerotic lesion, the question raised is whether the inflammatory response characterized by elevated circulating levels of biomarkers is an epiphenomenon or has a causal role. In other words, independently of cholesterol and regulators of blood pressure, could inflammatory biomarkers further report on different aspects of the pathogenic mechanisms underlying the disease?
As stated by Rao et al. [26], for the routine clinical use of a biomarker, several requirements must be accomplished: “1) the ability to control the standardization of the assay and variability of the measurement, 2) consistency in epidemiologic findings from prospective studies with clearly defined endpoints, 3) evidence that the marker adds to risk prediction over and above that already achievable through the use of established risk factors, 4) availability of population norms to guide interpretation of results, 5) generalizability to various population groups, 6) cost-effectiveness—the incremental cost of the test should be justified by a reduction in other costs and the indirect costs of a positive test should not be limiting” [7].
The list of putative biomarkers of inflammation has considerably grown during the last years, accompanying the extensive research on this area of knowledge. In 2003, a Centers for Disease Control and Prevention (CDC)/American Heart Association (AHA) scientific statement on markers of inflammation and CVD considered several inflammatory markers as potentially useful predictors of CVD (adhesion molecules; cytokines; acute-phase reactants, including C-reactive protein [CRP], serum amyloid A protein [SAA], and fibrinogen; white blood cell [WBC] count; and erythrocyte sedimentation rate [ESR]). However, the list of putative biomarkers of inflammation under evaluation during the last years is larger (Table 1.1).
Table 1.1
List of putative biomarkers of inflammation associated with cardiovascular disease
Acute–phase reactants |
High-sensitivity C-reactive protein (hsCRP) |
Fibrinogen |
Serum amyloid A (SAA) |
Pro–inflammatory cytokines |
Interleukin-1 (IL-1), IL-6, IL-18 |
Tumor necrosis factor alpha (TNF-α) |
Interferon gamma (IFN-γ) |
CD40/CD40 ligand |
Anti–inflammatory cytokines |
Interleukin (IL)-4, IL-10 |
Transforming growth factor (TGF-β) |
Adiponectin |
Other adipocytokines (leptin, resistin, visfatin) |
Cell adhesion molecules |
E-selectin, P-selectin |
Intercellular cell adhesion molecule-1 (ICAM-1) |
Vascular cell adhesion molecule-1 (VCAM-1) |
Platelet endothelial cell adhesion molecule-1 (PECAM-1) |
Chemokines |
Interleukin-8 (IL-8) |
Migration inhibitory factor (MIF) |
Monocyte chemoattractant-1 (MCP-1) |
Other molecules/mediators of inflammation |
Matrix metalloproteinases (MMPs) |
Myeloperoxidase (MPO) |
Myeloid-related protein (MRP) 8/14 |
White blood cell (WBC) count |
Erythrocyte sedimentation rate (ESR) |
Several other molecules have been associated with inflammation and CVD, but their primary nature is not inflammatory, including oxidative and carbonyl stress compounds; advanced lipoxidation end products (namely, malondialdehyde); advanced glycosylation end products (AGEs), such as plasma F2α-isoprostanes, advanced oxidation protein products, pentosidine, and carboxymethyl lysine; hemostatic and endothelial injury/dysfunction factors, including plasminogen activator inhibitor type 1, von Willebrand factor, and asymmetric dimethylarginine; homocysteine; lipid-associated markers, i.e., oxidized LDL and antibody to oxidized LDL, small dense LDL particles, lipoprotein (a), lipoprotein-associated phospholipase A2 (Lp-PLA2); heat shock protein (HSPs); and RANTES (regulated on activation, normal T cell expressed and secreted), among others.
Regarding clinical utility of putative inflammatory biomarkers of CV risk, it is important to consider some questions: does the biomarker add any information to that available from existing and well-established risk factors? Is it a suitable analyte? Is it stable in terms of diet influences and variations intra- and inter-day? Preferably, the biomarker should provide additional and independent information on cardiovascular risk; it should be easy to measure using standardized commercial assays with low variability and reasonably priced and not requiring specialized plasma collection or assay techniques. CRP has emerged as a robust, yet controversial clinical marker, since some of the previous requirements are accomplished.
This chapter starts exploring the most relevant data available and controversies and doubts concerning the putative use of CRP as a clinical biomarker of CV risk. Other molecule candidates to act as inflammatory biomarkers will be also debated.
High-Sensitivity C-Reactive Protein
High-sensitivity C-reactive protein (hsCRP) was first discovered in 1930, but its link to CHD was reported more than 60 years later. hsCRP is an acute-phase reactant mainly produced in hepatocytes in response to several cytokines, including IL-6 released from activated leukocytes in response to infection or trauma and from vascular SMCs during atherosclerosis lesion evolution. The role of CRP in atherosclerotic plaque formation is complex, acting in many cells involved in the process (Fig. 1.2) [27]. Although previous reports suggested a role of CRP as a surrogate of the underlying inflammatory process of atherothrombosis, accumulating evidence from in vitro and in vivo studies in clinical and experimental models robustly indicate a role of CRP as a proatherogenic factor [27–30].
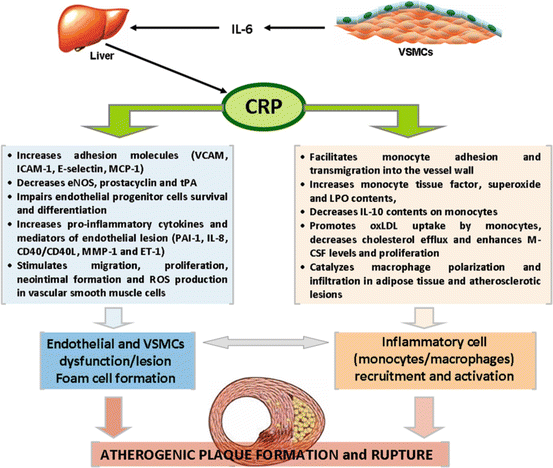

Fig. 1.2
Potential mechanisms of C-reactive protein (CRP) involvement in the pathogenesis of atherosclerotic plaque formation and rupture. While it remains uncertain whether CRP is directly involved in the pathogenesis of atherosclerosis or it is just a surrogate marker (an epiphenomenon) of other processes, several lines of evidence have been suggesting that CRP is localized within atherosclerotic lesions and exerts pro-inflammatory and proatherogenic effects
In brief, CRP induces endothelial cell activation and dysfunction by several distinct activities, including directly binding with highly atherogenic oxidized LDL-C (oxLDL); increasing adhesion molecules (VCAM-1, ICAM-1, E-selectin, MCP-1); decreasing eNOS, prostacyclin, and tPA; impairing endothelial progenitor cell (EPC) number and function; and increasing pro-inflammatory cytokines and other important mediators of endothelial lesion (such as PAI-1, IL-8, CD40/CD40L, MMP-1, and ET-1) underlying atherosclerosis; in addition, it facilitates monocyte adhesion and transmigration into the vessel wall, a critical early step in the atherosclerotic process, and promotes other important modifications on monocytes (increasing tissue factor, superoxide, and myeloperoxidase contents, decreasing IL-10 amounts, promoting oxLDL uptake, decreasing cholesterol efflux, and enhancing macrophage colony-stimulating factor [M-CSF] levels and proliferation); CRP also catalyzes macrophage polarization, which is a pro-inflammatory trigger in plaque deposition, leading to macrophage infiltration of both adipose tissue and atherosclerotic lesions [27–30].
Several lines of evidence have indicated that inflammation plays a central role in all stages of the atherothrombotic process. In clinical terms, translation of the atherosclerosis inflammatory hypothesis to practice has been based on observational evidence linking inflammatory biomarkers to the risk of future vascular events, namely, using hsCRP [31–33]. In fact, large-scale prospective studies demonstrate that CRP strongly and independently predicts adverse CV events, including MI, ischemic stroke, and sudden cardiac death [14, 33, 34]. The inclusion of CRP to classical cholesterol screening improves CV risk prediction independently of LDL-C, suggesting that increased CRP may identify asymptomatic individuals at high risk for future events, despite average cholesterol concentrations [33]. Furthermore, CRP concentration monitoring adds relevant prognostic information on CV risk at all LDL-C concentrations, but also at all levels of the FRS [33, 34]. As shown in the meta-analysis of Kaptoge et al., the magnitude of CV risk associated with a one-standard-deviation increase in hsCRP levels is at least as large as that associated with a one-standard-deviation increase in either total cholesterol or blood pressure [35]. Additionally, increased plasma CRP concentrations correlate with the components of MetS, such as central obesity, increased plasma triglyceride concentrations, low plasma concentrations of HDL-C, hypertension, and increased concentrations of blood glucose [33], and CRP contributes to risk prediction of MetS patients [36]. This evidence led to the development of the Reynolds Risk Score, which adds CRP to the FRS and improves global CV risk prediction in women by reclassifying >50 % of women considered at intermediate risk into higher- or lower-risk categories [37].
CRP was classified as an independent marker of CV risk by an expert panel assembled by the CDC and the AHA, as a way to improve risk stratification in populations’ primary prevention [38]. The panel recommended that global risk prediction in asymptomatic individuals deemed at intermediate risk for CVD by classical risk factors should include CRP measurement and the cutoff points of <1 mg/L for low-risk and >3 mg/L for high-risk individuals.
Further than being used as an adjunctive tool in risk prediction and reclassification, there is interest in using hsCRP levels to select patients for statin initiation and to tailor intensity of therapy. Statins reduce hsCRP in an LDL-independent manner, and the benefits are superior in patients with inflammation [39]; the lower the hsCRP levels, the lower the risk [40, 41]. These evidences raised the question of whether patients that do not meet criteria for statin prescription (given the low/average LDL-C concentrations) would benefit from that medication if they had hsCRP > 2 mg/L, indicative of an enhanced inflammatory response, thus suggesting that statins could have a dual influence: reduction of LDL-C levels and direct anti-inflammatory effects. These questions/hypotheses were the basis for the JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) [42], which enrolled 17,802 men and women with no evidence of CV disease and average or low LDL-C contents, for testing the putative benefit of rosuvastatin (20 mg po daily) treatment. JUPITER trial showed a major reduction in CV events (54 % in MI and 51 % in ischemic stroke) and in all-cause mortality (20 %), as well as in need for bypass surgery or angioplasty (46 %), with an overall 44 % relative risk reduction for the primary endpoint of major arterial vascular events. Results were identical between several subpopulations in all ethnic groups, including women vs. men, elderly, as well as with and without arterial hypertension, obesity, or MetS [42–44]. As recently commended [45], JUPITER trial also showed that there was a significant reduction in venous thromboembolism (43 %), and the maximum levels of risk reduction were found in those who achieved low hsCRP levels. Magnitude of hsCRP reduction could not be predicted on the basis of the magnitude of LDL-C reduction, and the reduction of absolute risk of events for both the rosuvastatin-treated and placebo-treated (control) groups was greater among those with higher levels of CRP at study entry, an effect not observed for LDL-C. Genetic determinants of rosuvastatin-induced LDL-C reduction were found to differ from the genetic determinants of rosuvastatin-induced CRP reduction, altogether suggesting that at least part of the benefits of statin therapy were due to anti-inflammatory effects independent of LDL-C reduction. All those strong evidences coming from JUPITER trial, including the smaller number needed to treat (NNT) found for subjects with low LDL-C levels and elevated hsCRP concentrations (when compared with primary prevention patients under treatment of dyslipidemia or arterial hypertension) [46], had impacted the spectrum of patients candidate for statin therapy according to the FDA, as well as to other several national authorities, now including patients with elevated hsCRP levels and at least one additional risk factor, independently of high or average LDL-C levels.
Despite several strong indications coming from that trial, highlighting a statin benefit that goes beyond the effect on LDL-C reduction, additional studies were recommended to clearly test the hypothesis that directly targeting inflammation will improve vascular outcomes. The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) and the Cardiovascular Inflammation Reduction Trial (CIRT) have recently started and will evaluate, respectively, a human monoclonal antibody that targets human interleukin-1β (IL-1β) and low-dose methotrexate, in order to reduce cardiovascular event rates, due to direct anti-inflammatory effects [47, 48]. The results of these trials are expected with great curiosity, as they could be essential to define new algorithms to improve CV risk prediction and to gather information that could serve as basis to define new drugs targeting the machinery of inflammation.
Emergent Biomarkers
Other Acute-Phase Reactants
Serum amyloid A (SAA) protein and fibrinogen, like CRP, are acute-phase reactants generated downstream of IL-6 in the liver, as part of the acute-phase response, reflecting the intensity of cytokine activation.
Fibrinogen
Fibrinogen influences endothelial function, thrombosis, and inflammation and has been indicated as an independent variable contributing to CV risk. In brief, fibrinogen forms the substrate for thrombin (leading to platelet aggregation), modulates endothelial function, and promotes SMC proliferation and migration [49]. Several epidemiologic studies demonstrate that fibrinogen concentrations predict future risk of MI and stroke. However, it seems to be a less potent predictor of CV events than CRP [50]. Whether or not fibrinogen is causally involved in atherothrombogenesis remains to be elucidated.
Serum Amyloid A (SAA)
SAA protein, like CRP, is an acute-phase protein synthesized in the liver in response to infection, inflammation, injury, or stress. It has been linked to atherosclerosis, namely, because it is secreted as the predominant apolipoprotein on plasma HDL cholesterol particles, where it seems to replace apolipoprotein A-I, thus changing HDL-mediated cholesterol delivery to cells [51].
The more rapid response of SAA than other nonspecific inflammatory markers, such as CRP, has suggested that it could be a better marker of disease. SAA has also been shown to be a predictor of CV events [52]. However, some studies suggest that this relationship may be dependent on other risk factors [32], indicating that the independent predictive value of SAA for CAD and CV events remains unclear, deserving further studies.
Cytokines
Cytokines are key in regulating inflammatory and immune responses and have a pivotal role in controlling the innate and the adaptive immunity. Pathogenesis of atherosclerosis involves a complex interplay between cytokines, chemokines, and adhesion molecules, leading to monocyte infiltration and multiple other leukocyte responses within the arterial wall. A variety of plasma inflammatory markers have been shown to predict future CV risk. In addition, they may be useful for risk stratification and also to identify patients who might benefit from targeted therapy. Cytokines are classified according to their pro- or anti-inflammatory activities. The balance between pro- and anti-inflammatory cytokines has emerged as a major determinant of plaque stability [53].
Pro-inflammatory Cytokines
Several inflammatory cytokines have also been investigated as markers of CV risk, including TNF-α, IL-6, and CD40/CD40 ligand.
Tumor Necrosis Factor Alpha (TNF-α)
TNF-α is a cytokine primarily produced by macrophages, endothelial cells, and SMCs of atherosclerotic arteries and has been shown to have several pro-inflammatory properties, including induction of expression of cellular adhesion molecules, surface leukocyte adhesion molecules, chemokines, other cytokines, and growth factors, as well as proangiogenic activity [54]. TNF-α activities affect atherosclerotic process and have been implicated in metabolic disorders, such as obesity and insulin resistance [54, 55]. Increased plasma concentrations of TNF-α have been associated with increased risk of CV events, namely, in stable patients after MI, as it was demonstrated in the Cholesterol and Recurrent Events (CARE) trial [56].
Interleukin-6 (IL-6)
IL-6 is produced by hepatocytes, endothelial cells, fibroblasts, phagocytes, neutrophils, and lymphocytes, among other cell types. This pleiotropic cytokine has a broad range of functions and regulates several cellular processes, including growth, differentiation, angiogenesis, and healing. The precise role of IL-6 in the evolution of atherosclerosis lesions remains uncertain, but several important activities/effects of IL-6 have been described, namely, in ApoE knockout mice, including stimulation of synthesis and secretion of CRP and enhancement of fatty lesion development [57]. Increased levels of IL-6 seem to be predictive of future CV events, as suggested in the Physicians’ Health Study (PHS) [58].
CD40/CD40 Ligand
CD40 ligand (CD40L) is a transmembrane protein of the TNF family that links to its receptor (CD40) and has a role in the inflammatory processes underlying atherosclerosis, plaque destabilization, and thrombosis. In fact, CD40/CD40L, expressed in a variety of immune and vascular cells, regulates platelet-dependent responses that contribute to atherothrombosis, activate endothelial cells, and in vitro promote expression of adhesion molecules, pro-inflammatory cytokines, and chemokines [59]. Soluble CD40L levels have been indicated as predictive of CV events (MI and stroke) and death in some populations [60].
Anti-inflammatory Cytokines
Interleukin-4 (IL-4) and IL-10
IL-4 and IL-10 are pleiotropic cytokines produced by Th2 lymphocytes and by other types of immune cells that have been associated with anti-inflammatory activities, mostly in mouse models of atherosclerosis. While decreased IL-10 levels have been reported in patients with acute CV events [61], the association of IL-4 levels with CVD is debatable as IL-4 may also play a role in atherosclerosis through induction of inflammatory responses (it is worth to say that increased IL-4 levels were found in patients with CAD) [62].
Transforming Growth Factor Beta (TGF-β)
TGF-β is a potent anti-inflammatory cytokine that plays a pivotal role in the maintenance of normal blood vessel wall architecture and protects against vulnerability to atherosclerosis. TGF-β isoforms 1, 2, and 3 are mainly expressed by SMCs and modulate vascular development and remodeling and determine the extent to which developing atherosclerotic lesions are stabilized [63]. Decreased levels of TGF-β1, as well as genetic polymorphisms and defective TGF-β signaling, have been reported in patients with CVD [64, 65].
Adiponectin
Adiponectin is an adipocytokine produced by adipocytes that exerts anti-inflammatory and antiatherogenic effects, having a protective role in CV terms [66]. It reduces TNF-α-stimulated expression of E-selectin, NF-κB, VCAM-1, and IL-8 and regulates monocyte adhesion to endothelium and endothelial nitric oxide synthase (eNOS) activity. Adiponectin also has insulin-sensitizing effects, and its secretion diminishes as adipose tissue mass increases. It is suggested that adiponectin contributes to the relationship between obesity, insulin resistance, and CV disease. Its concentrations are inversely associated with CVD incidence in most of the studies. In the PHS, there was a robust inverse relationship between total adiponectin and incident CHD, even after adjustment for traditional risk factors, while high levels of adiponectin have been associated with lower risk for CV events [67, 68].
These observations suggest that there is promise for the application of adiponectin and other cytokines as predictors of CVD risk. However, since the associations are complex, a more complete understanding of the exact role played by these emergent biomarkers in disease’s pathophysiology is required, as well as stronger evidences from larger clinical studies without confounding factors and after proper adjustment for traditional risk factors.
Cell Adhesion Molecules
Due to their central role in the recruitment of inflammatory cells to the site of atheroma development, the cell adhesion molecules (CAMs) are promising candidates to reflect underlying vascular inflammation. E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intercellular cell adhesion molecule-1 (ICAM-1) are all members of the cellular adhesion molecule family, each having a plasma-soluble form, which can serve as a surrogate marker for increased expression of CAMs on vascular endothelial cells, reflecting inflammation and activation of endothelial cells [69].
E-Selectin
E-selectin is the most interesting form of the selectin family, which also includes L and P selectins. E-selectin promotes the interaction between endothelial cells, where it is expressed, to leukocytes. While increased E-selectin levels have been observed in some studies with CVD populations, other reports showed divergent results; at the moment, the prognostic value of E-selectin remains to be clearly defined [69, 70].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


