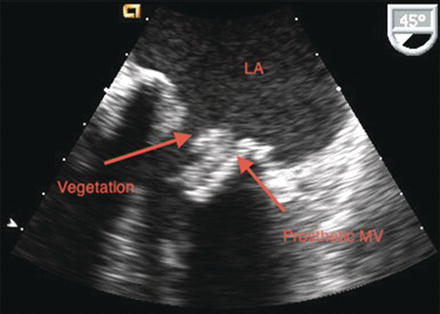
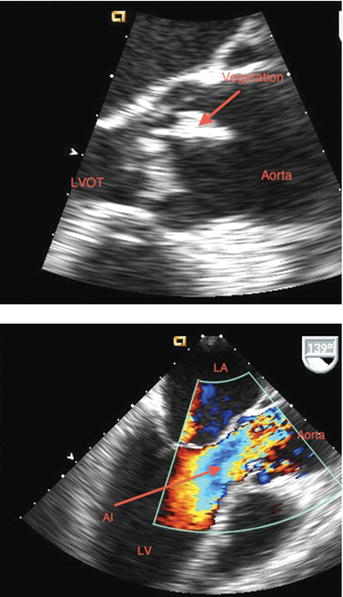
Figure 25.1 TEE of aortic valve endocarditis with aortic regurgitation. AI, aortic regurgitation; LVOT, left ventricular outflow tract. (See Color plate 25.1).
25.2 PATHOPHYSIOLOGY
Developing valvular IE is a complex process involving multiple interacting components. In order for a vegetation to form, the valve surface must first be altered or damaged. Valve surface damage often results in blood flow turbulence and is frequently followed by platelet and fibrin deposition with eventual formation of nonbacterial thrombotic endocarditis (NTE). Bacteria may then colonize the lesion after formation of NTE. The continued proliferation of bacteria and deposition of platelets and fibrin result in a vegetation that can embolize peripherally and result in embolic phenomena. Often, these vegetations create a biofilm, thus making them impermeable to the body’s defenses as well as antibiotic therapy.
Table 25.1 Predisposing Conditions Associated with Infective Endocarditis (IE).
| Predisposing conditions |
| Chronic intravenous access Congenital heart defects Intravenous drug use Invasive procedures within 60 days Native valve predisposition Pacemaker/intracardiac defibrillators Previous IE |
The clinical features of IE are a consequence of (1) local cardiac destruction, (2) systemic embolization of the vegetation, and (3) deposition of circulating immune complexes. Intracardiac complications vary from minimal tissue damage to extensive valvular destruction and significant hemodynamic deterioration. Common sites of emboli include the central nervous system, spleen, lung, and skin. Immune complexes can deposit in the glomerular basement membrane, resulting in glomerulonephritis.
Table 25.2 Clinical Findings in Patients with Definite Infective Endocarditis*.
| Clinical finding | Percentage of cases (%) |
| Fever, temperature >38 °C | 96 |
| New murmur | 48 |
| Hematuria | 26 |
| Worsening of old murmur | 20 |
| Vascular embolic event | 17 |
| Splenomegaly | 11 |
| Splinter hemorrhages | 7 |
| Conjunctival hemorrhage | 5 |
| Janeway lesions | 5 |
| Osler nodes | 3 |
| Roth spots | 2 |
*Adapted from Murdoch DR, Corey R, Hoen B, et al: Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis – Prospective Cohort Study. Arch Intern Med 2009;169:463.
25.3 CLINICAL FEATURES
The period of time from the onset of bacteremia to symptoms of IE is generally less than two weeks, although this can vary based on the infecting organism. The most common clinical finding of IE is fever observed in more than 95% of patients (Table 25.2). Constitutional symptoms result from cytokine release and include arthralgias, myalgias, anorexia, and malaise. The risk of systemic emboli increases with vegetations greater than 10 mm. Embolization risk decreases shortly after the administration of antibiotics. Stroke occurs in 15–20% of patients and most commonly involves the middle cerebral artery. Congestive heart failure results from valvular destruction, fistula formation, and myocarditis. Renal insufficiency can result from the deposition of circulating immune complexes. Although classically described with IE, peripheral manifestations that can result from either systemic emboli or immune complex deposition are seen in fewer than 10% of IE cases. Such manifestations include petechiae, splinter hemorrhages (linear streaks in the proximal nail bed), Osler nodes (tender subcutaneous nodules in the pulp of digits), Janeway lesions (small erythematous nontender lesions on the palms and soles), and Roth spots (oval retinal hemorrhages with a pale center).
Table 25.3 Definition of Infective Endocarditis (IE) According to the Modified Duke Criteria*.
| Definite IE Pathologic criteria 1. Presence of microorganisms by culture or embolized vegetation or an intracardiac abscess or 2. Vegetation or abscess confirmed by histology showing active endocarditis. Clinical criteria can be confirmed by ANY of the following: a. 2 major criteria; or b. 1 major criterion and 3 minor criteria; or c. 5 minor criteria Possible IE can be considered by ANY of the following a. 1 major criterion and 1 minor criterion; or b. 3 minor criteria Diagnosis of IE can be withdrawn by ANY of the following 1. Alternate diagnosis explaining evidence for IE; or 2. Resolution of IE via antibiotic therapy for ≤4 days; or 3. No evidence of IE at surgery with antibiotic therapy for ≤4 days; or 4. Does not meet criteria for possible IE, as above |
*See Table 25.4 for definitions of major and minor criterion.
25.4 DIAGNOSIS
Infective endocarditis can be an imprecise diagnosis as bacteremia often occurs without endocardial involvement and IE can occur with negative blood cultures. There are numerous mimickers of IE including, but not limited to, systemic lupus erythematous, thrombotic thrombocytopenic purpura, carcinoid syndrome, and rheumatic fever. Therefore, clinicians must maintain a high-index of suspicion for IE in any patient with a cardiac murmur, organic valvular disease, congenital heart disease, or a prosthetic valve who presents with fever, anemia, hematuria, or other physical findings suggestive of IE. The Modified Duke Criteria provides a highly sensitive and specific diagnostic strategy to aid in diagnosis (Table 25.3, Table 25.4).
Echocardiography plays an important role in the diagnosis of IE. However, it should not be used indiscriminately as a screening tool in patients with fever and positive blood cultures who are unlikely to have IE. Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) when applied in the correct clinical setting are helpful in diagnosis. The specificity of TTE in vegetation detection is approximately 98% while the sensitivity is 30–40%. If the pre-test probability for IE is low, TTE offers a cost-effective and clinically appropriate test for evaluation of native valve endocarditis. For concerns of prosthetic valve endocarditis or patients with a high pre-test probability of native valve endocarditis, TEE is an appropriate initial imaging modality. The Modified Duke criteria recommend TEE for patients with prosthetic heart valves, rated at least “possible” IE, or complicated IE (i.e. paravalvular abscess) while a TTE is appropriate for all other patients.
Table 25.4 Definition of Terms used in the Modified Duke Criteria*.
| Major criteria | 1. Blood cultures positive for IE from 2 separate cultures a. Microorganisms such as Viridans streptococci, Streptococcus bovis, HACEK group, Staphylococcus aureus; or community-acquired enterococci with no other source. Blood cultures must be drawn >12 h apart; or all of 3 or a majority of ≥4 separate cultures of blood (with first and last sample drawn at least 1 h apart) b. Single positive blood culture for Coxiella burnetti or antiphase IgG antibody titer > 1:800 2. Evidence of endocardial involvement 3. Echocardiogram which displays ANY of the following a. Evidence of vavular mass or mass noted on supporting structure or on implanted material
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|