(1)
University of Ottawa The Ottawa Hospital, Ottawa, ON, Canada
Infective endocarditis (IE) most often results from bacterial infection, but infections caused by fungi, Coxiella, or Chlamydia are not rare. Infection usually involves heart valves not always previously known to be abnormal, in particular a bicuspid aortic valve, mitral valve prolapse, or (rarely) a septal defect or ventricular aneurysm. Coarctation of the aorta, patent ductus arteriosus, aneurysms, or arteriovenous shunts may be the site of infective endarteritis. Prosthetic valves may be involved, and infection at the site of implantation of foreign material including devices poses a particularly difficult problem.
The old fashioned terms acute and subacute bacterial endocarditis (SBE) are still clinically useful, although they no longer hold prominence because pathogens such as Staphylococcus aureus and streptococci can cause either fulminant or indolent disease in different patients.
The term SBE or subacute IE used in a patient who is not critically ill refers to a subacute syndrome, with minimal signs of toxicity, which is usually caused by viridans streptococci, enterococci, coagulase-negative staphylococci, or gram-negative coccobacilli. These organisms cause a slow, low-grade infection that evolves clinically over weeks to months, thus allowing the clinician to delay therapy for a few days while awaiting the results of blood cultures and other diagnostic tests.
Acute IE is accompanied by marked toxicity with progression over days to a few weeks resulting in valvular destruction, hemodynamic deterioration, heart failure (HF), and metastatic infection and is caused mainly by S. aureus, which carries about a 40 % rate of HF.
Rheumatic valvular heart disease is now uncommon in developing countries, and IE is encountered mainly in patients with prosthetic heart valves, bicuspid aortic valves, particularly in men over age 60, and degenerative valvular disease (aortic sclerosis). Mitral valve prolapse (MVP) accounts for approximately 18 % of native valve IE, with an increased risk in men older than 45. The risk of IE in patients with MVP is significant mainly if a regurgitant murmur is heard or if there is documented thickening of valve leaflets >5 mm. Intravenous (IV) drug abusers represent a special group with right-sided IE.
Classification and Diagnosis
A logical classification of IE is as follows:
Native valve IE; acute or subacute presentation.
Prosthetic valve IE.
Right-sided endocarditis, observed particularly in IV drug users.
Culture-negative IE.
The European Society of Cardiology (ESC 2009) provided a statement: “The epidemiology of IE has changed, now more often affecting elderly patients and occurring as a result of health care-associated procedures. IE can be categorized as left-sided native valve IE, left-sided prosthetic valve IE, right-sided IE, or device-related IE.”
Diagnostic Guidelines
Diagnostic criteria are as follows:
Conformation of persistent bacteremia resulting from organisms.
Evidence of cardiac valvular involvement: documentation of vegetation, new murmur of valvular regurgitation, or paravalvular abscess.
Supporting findings include: fever, risk factors for IE, vascular or immune complex phenomena, or intermittent bacteremia or fungemia.
The diagnosis of IE requires a high index of suspicion. The condition should be considered and carefully excluded in all patients with a heart murmur and pyrexia of undetermined origin. The Duke criteria utilize echocardiographic findings as a major criterion for diagnosis and have merit for diagnosis of native valve endocarditis; the utility of the Duke criteria has not yet been adequately assessed, however, for suspected prosthetic valve endocarditis. Diagnosis is made in the majority by blood cultures and echocardiography.
Echocardiography: Transesophageal echocardiography (TEE) is superior to transthoracic assessment in the search for infected vegetations located on heart valves and is crucial for the diagnosis of endocarditis. See Fig. 16-1.
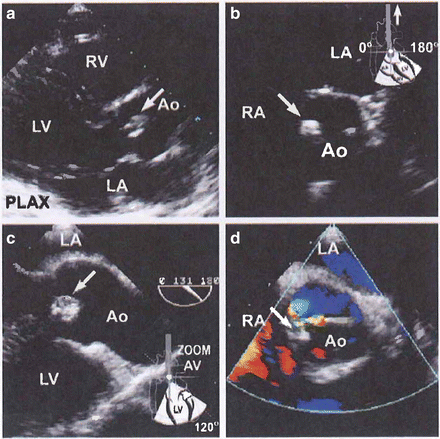

Fig. 16-1.
A 40-year-old heroin user presenting with a leg pain, visual disturbances, fever, and a murmur. Transthoracic still frames showing a vegetation on the tip of an aortic cusp (arrow, a). This was believed responsible for the patient’s extracardiac complications. No tricuspid vegetations were observed. Transesophageal images revealed a circumscribed vegetation (arrows, c, d). Mild aortic regurgitation was seen on Color Doppler examination (d). Reproduced with permission from Anavekar NS, Averbach M, Bulwer BE: Infective Endocarditis. In: Solomon SD, Bulwer BE, editors. Essential Echocardiography: A Practical Handbook. Totowa, NJ: Humana Press, 2007; p. 292. With kind permission of Springer Science + Business Media.
Transthoracic two-dimensional Doppler echocardiography gives poor detection of prosthetic heart valves, especially in the mitral position and of calcific sclerotic native valves. Vegetations that are less than 5 mm, 6–10 mm, or greater than 10 mm are observed in 25 %, 65 %, and 70 %, respectively, by transthoracic technique. This is 100 % for all lesions using TEE.
Two-dimensional transthoracic endocardiography (TTE) can miss 25 % of vegetations <10 mm and 75 % of those <5 mm. (TEE) is more sensitive than TTE for detecting vegetations and cardiac abscess.
High-risk echocardiographic features include
Large and/or mobile vegetations.
Valvular insufficiency.
Suggestion of perivalvular extension.
Secondary ventricular dysfunction.
Need for surgery
Heart failure is an immediate indication. Features that suggest potential need for surgical intervention (Baddour et al./AHA 2005) are as follows:
Abscess formation.
Staph infection.
Persistent vegetation after systemic embolization.
Anterior mitral leaflet vegetation, particularly with size >10 mm.
One or more embolic events during first 2 weeks of antimicrobial therapy.
Increase in vegetation size >1.5 cm despite appropriate antimicrobial therapy.
Acute aortic or mitral insufficiency with signs of ventricular failure.
Valve perforation or rupture, valvular dehiscence, or fistula.
Large abscess or extension of abscess despite appropriate antimicrobial therapy.
New heart block.
Combination of large vegetation size and positive antiphospholipid antibody.
Precipitating and predisposing factors
If prior to dental work, the usual organism producing IE is S. viridans or, rarely, S. faecalis. If an acute presentation emerges after dental work, one must suspect Staphylococcus or the extremely rare Fusobacterium, which is not uncommon in gingival crevices and the oropharynx.
With genitourinary instrumentation or in other surgical procedures, gram-negative bacteria are the rule.
Prosthetic heart valve.
Narcotic addicts: mainly right heart endocarditis, owing to S. aureus, Pseudomonas aeruginosa, P. cepacia, and Serratia marcescens.
Blood cultures: Adequate cultures and as wide as possible a range of sensitivities must be obtained. Approximately 90 % of the causative organisms can be isolated if there has been no previous antibiotic therapy. The past two decades have seen an increasing incidence of staphylococci and enterococci, often resistant to penicillin. The incidence of gram-negative organisms has also increased. If the organism is to be isolated at all, four to six blood cultures carry a 98 % chance of success.
Three separate sets of blood cultures should be taken, each from a separate venipuncture site, over 24 h. Ten milliliters of blood drawn should be put in each of two blood culture bottles, one containing aerobic and the other anaerobic medium (Towns and Reller 2002).
If the presentation is acute and the patient’s status is critical with a high suspicion of S. aureus infection, another view is to take four blood cultures over a period of 1–2 h, after which antibiotic treatment should begin and should on no account be withheld pending a bacteriologic diagnosis. The presence of echocardiographically visible vegetations greatly increases the urgency of commencing treatment, as does the presence of infection on prosthetic valves.
Aids in identifying the organism:
Cultures must be incubated both aerobically and anaerobically; the latter is necessary especially for Bacteroides and anaerobic streptococci.
Serological tests (complement fixation tests (CFTs)) are of value in patients with Brucella, Candida, Cryptococcus, Coxiella, or Chlamydia.
Examination of a Gram stain of the “buffy” coat of the peripheral blood.
In cases other than group A Streptococcus, it is advisable to monitor the serum bactericidal titer (SBT) 1:8 or higher, the minimum inhibitory concentration (MIC), and the minimum bactericidal concentration (MBC).
A discussion of the case with the microbiologist is often helpful. Some organisms such as Haemophilus influenzae and variants of streptococci require enriched media. Neisseria gonorrhoeae and N. meningitidis require 5–10 % of CO2, and Pseudomonas grows poorly in unvented bottles. Fungi require a medium containing broth and soft agar and are seldom identified by culture. The culture of an arterial embolus may reveal a fungal etiology.
Therapy
Initial choice of appropriate antibiotic prior to laboratory determination of the infecting organism is guided by the following parameters:
1.
Native valve: a subacute presentation, SBE, is caused by S. viridans in approximately 80 %, S. faecalis in 10 %, and other organisms in 10 %.
2.
Native valve, elderly endocarditis: S. faecalis is commonly seen, but S. viridans is implicated in about 50 % of cases.
3.
Prosthetic valve endocarditis.
Early infection after operation is usually caused by Staphylococcus epidermidis or S. aureus.
Late after operation the organisms are similar to those seen in SBE or acute endocarditis with the additional probability of fungal infection, but S. epidermidis is not uncommon. Following abdominal surgery, gram-negative and anaerobic infections are not uncommon.
4.
Acute bacterial endocarditis (ABE) is usually caused by S. aureus.
5.
Endocarditis in narcotic addicts: right-sided endocarditis.
6.
Culture-negative endocarditis is often caused by:
The usual bacterial organisms, which are masked by previous antibiotic therapy.
Slow-growing penicillin-sensitive streptococci with fastidious nutritional tastes.
Coxiella and Chlamydia.
S. aureus Endocarditis
This most harmful organism accounts for nearly all cases of native valve acute bacterial endocarditis and approximately 50 % of prosthetic valve IE.
For penicillinase-producing staphylococci, nafcillin, oxacillin, or flucloxacillin with the optional addition of gentamicin is given for 6 weeks, the latter for only 1 week (see Table 16-1). An aminoglycoside is not added in the United Kingdom. Other regimens advised for penicillinase-producing staphylococci include:
Table 16-1
Treatment of Staphylococcus aureus Endocarditis
Type | Antibiotic | Dosage |
|---|---|---|
Native valve | Nafcillin + | 2 g IV every 4 h for 6 weeks |
Optional | 1–1.4 mg/kg IV every 8 h for 1 week | |
Gentamicin | ||
Methicillin-resistant staphylococci | Vancomycin | 15 mg/kg IV every 12 h for 6 weeks |
Prosthetic valve | Nafcillin + | 2 g every 4 h for >6 weeks |
Rifampin | 300 mg orally every 8 h for 6 weeks | |
Gentamicin | 1–1.4 mg/kg IV every 8 h for 2 weeks | |
Methicillin-resistant staphylococci | Vancomycin + | 15 mg/kg IV every 12 h for 6 weeks |
Rifampin | 300 mg orally every 8 h for >6 weeks | |
Gentamicin | 1–1.4 mg/kg IV every 8 h for 2 weeks |
Vancomycin
Cephalosporins: cephalothin, cephradine, cefuroxime
Rifampin plus aminoglycoside; rifampin plus cloxacillin; rifampin plus vancomycin
Clindamycin and cephalosporin
Vancomycin and cephalothin are effective alternatives when penicillin is contraindicated. Cephalothin is more active than other cephalosporins against S. aureus. Clindamycin is relatively effective but is not advisable because it is bacteriostatic or less bactericidal than penicillin or cephalosporins; also, pseudomembranous colitis may supervene. If metastatic infection is present, rifampin is usually added at a dose of 600–1,200 mg daily and continued until abscesses are drained and excised. For methicillin-resistant staphylococci, vancomycin 1 g every 12 h is the treatment of choice. Care should be taken in patients over age 65 years and/or those with renal impairment or eighth nerve dysfunction. Vancomycin serum levels should be maintained at <50 μg/mL. There appears to be no advantage in adding an aminoglycoside or rifampin. If vancomycin is contraindicated, no suitable alternatives have been tested: trimethoprim–sulfamethoxazole (Bactrim, Septra) has been used with some success. Rifampin must not be used alone because resistant strains quickly emerge. Fusidic acid 500 mg four times daily with rifampin has been used and provides a reasonable alternative.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


